|
¡@
What is Silicon?

Historical Information of Silicon
- Silicon was discovered by Jons Jacob Berzelius, at
Sweden in 1842.The origin of the name silicon came from the Latin word 'silicis' meaning
'flint'
- Silicon, which is the second most abundant element by
weight in the earth crust , is a well known element .It occurs very widely , as silica SiO2 (sand and quartz), and in a wide variety of silicate minerals and
clays.
- It is present in the sun and stars and is a principal
component of a class of meteorites
- known as aerolites . Silicon make up 25.7 % of the
earth crust by weight, and is the second most abundant element, exceeded only by oxygen .
It is found largely as silicon oxides such as sand (silica), quartz, rock crystal,
amethyst, agate, flint, jasper and opal. Silicon is found also in minerals such as
asbestos , clay and mica.
- ¡@
 | Sun (relative to H=1x1012):
4.47x107 |
 | Earth crust/p.p.m.: 277 000 |
 | Alantic surface: 0.03 |
 | Alantic deep: 0.82 |
 | Pacific deep: 4.09 |
 | Residence time/years: 30 000 |
 | Classification : recycled |
 | Oxidation state: IV |
- ¡@
- Silicon is important in plant and animal life. Diatoms
in both fresh and salt water extract silica from the water to use as a component of their
cell walls. Silicon is an important ingredient in steel. Silicon carbide is one of the
most important abrasives. Workers in environments where silicaceous dust is breathed may
develop a serious lung disease known as silicosis.
- Hydrolysis and condensation of substituted
chlorosilanes can be used to produce a very great number of polymeric products, or
silicones. These range from liquids to hard, glasslike solids with many useful properties.
The uses of Silicon will be discussed later in Section
- Silicon is a dark grey with bluish tinge substance ,
with its standard state as solid
- At 298K . Elemental silicon transmits more than 95% of
all wavelengths of infrared and has been used in lasers to produce coherent light at
465nm.
¡@
| Melting point/K |
1683 |
| Boiling point/K |
2628 |
| Latent heat of fusion/kJ
mol-1 |
39.6 |
| Latent heat of vaporisation/kJ
mol-1 |
383.3 |
| Density/kg m-3 |
2329[293K] ; 2525[liquid at
m.p.] |
| Thermal
conductivity/Wm-1K-1 |
148 [300 K] |
| ¡@ |
¡@ |
¡@

¡@
 Electronegativities can be used to obtain a qualitative , if not a
quantitative , measure of the relative charges on atoms in molecules. And because the
charge distribution in a molecule affects the interactions of the molecule with other
polar groups and ions , electronegativities give information regarding relative chemical
reactivity . For example , the relative electronegativities of carbon and silicon indicate
that the hydrogen atoms in silicon hydrides should be considerably more negatively charged
than those hydrocarbons. Electronegativities can be used to obtain a qualitative , if not a
quantitative , measure of the relative charges on atoms in molecules. And because the
charge distribution in a molecule affects the interactions of the molecule with other
polar groups and ions , electronegativities give information regarding relative chemical
reactivity . For example , the relative electronegativities of carbon and silicon indicate
that the hydrogen atoms in silicon hydrides should be considerably more negatively charged
than those hydrocarbons.
Si H C H
1.8 2.1 2.5 2.1
¡@
Chemical properties
- Radii/pm: Si 4+ 26; atomic 117; covalent 117; van der
Waals 200;
- Si 4+271
¡@
A brief introduction of
silicon compound
Oxides of silicon:
- There are two oxides of silicon , SiO and SiO2
.
- Silicon monoxide is thought to be form by high
temperature reduction of SiO2 with Si , but it's existence at room temperature
is in doubt :
- ¡@
-
SiO2 + Si à 2SiO
- ¡@
- Silicon dioxide SiO2 ( silica ) is widely
found as sand and quartz . Group 14 elements typically form 4 bonds . However , silicon
cannot form p£k¡V p£kdouble bonds . Thus , SiO2 forms an infinite
three-dimentional structure and SiO2 is a high melting solid . SiO2
exists in at least 12 different forms . The main one are quartz , tridymite and
cristobalite , each of which has different structures at high and low temperatures . Pure
SiO2 is colourless , but traces of other metals may colour it giving
semi-precious gemstones such as amethyst ( violet) , rose quartz ( pink ) and non-precious
material such as flint ( often black due to carbon ) .
- The structure of silicon dioxide is as below :
¡@

¡@
- Each Si is tetrahedrally surrounded by four O atoms .
Each corner is shared with another tetrahedron , thus give an infinite array .
Silicates
- Silicate is a salt of silicic acid . In mineralogical
chemistry the silicates include ; the unisilicates or orthosilicates ( fig i ) , salts of
orthosilicic acid ; the bisilicates or metasilicates , salts of metasilicic acid ; the
polysilicates or acid silicates , salts of the polysilicic acids ; the basic silicates or
subsilicates , in which the equivalent of base is greater than would be required to
neutralize the acid ; and the hydrous silicates , including the zeolites and many hydrated
decomposition products .
- The followings are the examples of structure of
different silicates :
- ¡@
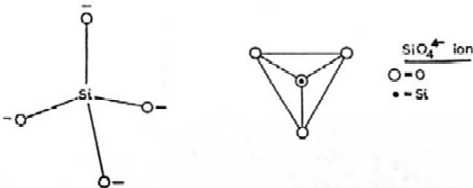
( i ) structure of orthosilicates
¡@
( ii ) structure of disilicates
¡@
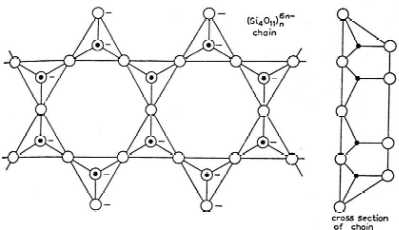
( iii ) structure of chain silicates
- ¡@
- ¡@

( iv ) structure of pyroxenes
- ¡@

( v ) structure of sheet silicates
- ¡@

( vi ) structure of cyclic silicates
-
- ¡@
- About 95% of earth crust is composed of silicate
minerals , alumino-silicate clays or silica . These make up the bulk of all rocks , sands
, and their breakdown products clays and soil . Many building materials are silicates :
granite , slates , bricks and cement . Ceramics and glass are also silicates. The reasons
for the high abundant of silicate in the earth is , during the cooling of the earth , the
lighter silicate materials crystallzed and floated to the surface , resulting in the
concentration of silicates in the earth crust .
Silicon tetrachloride
- Silicon tetrachloride SiCl4 is volatile
molecular compound . It is prepared by direct reaction of the elements :
Si + 2Cl2 à SiCl4
- Much of the production of the tetrachloride is
destined for purification by distillation (refer to the section : extraction of silicon)
followed by reduction with hydrogen, magnesium or zinc to produce semiconductor-grade
silicon :
- ¡@
SiCl4 + 2Mg à Si + MgCl2
Or
SiCl4 + 2H2à Si +4HCl
- Silicon halides are mild Lewis acids . They display
this character when they add one or two ligands to yield complexes with a five-coordinate
or six-coordinate central atom :
SiF4 + 2F- à [SiF6 ]2-
¡@
Back to top
|