

The diagram above is obtained from here using the original graph from EKA Chemicals. An
explanation of how to interprete
mutual solubility diagrams is in How to Design Fractional Crystallization Processes.
Also the book "Aqueous Solutions and the Phase Diagram" by FREDERICK FIELD PURDON
and VICTOR WALLACE SLATER, is very useful reading. It is available in Google Books.
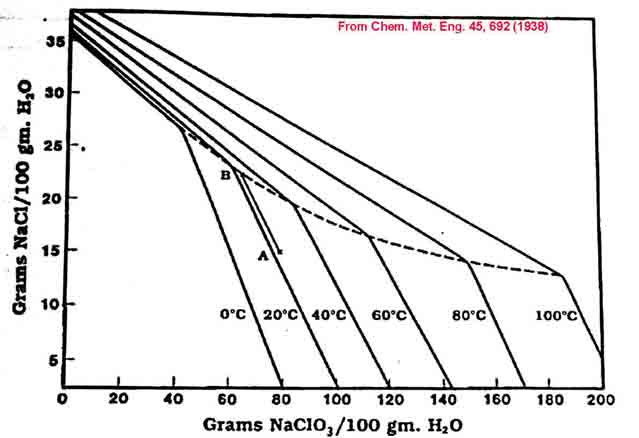
When you start off your cell for the first time you will be at 26% NaCl on the graph. If you do not add any Chloride solution as the cell is running and you run your cell for the recommended amount of time given by the run time formula, you will be left with about 10g/l00ml (100g/l) of Chloride in the cell and the rest of the Chloride (25g/100ml) will have been converted into 106.5/58.5 * 25g = 45.5g Chlorate per 100ml solution. This in approximately point A on the graph, 64.7% water, 6.3% NaCl and 29% NaClO3. As you can see you will not get any Chlorate out by cooling. If you boil off about 14.8% of the water you will get to point C on the graph. If you now cool your solution to Zero (thatís point B) you will get about 11% of the Chlorate to ppt.
If you add Sodium Chloride to the cell as it works you can increase the Chlorate concentration so that you will get a Chlorate ppt. when the cell is finished. Remember that if you do not take out the Chlorate this time around you will get out more the next time you harvest Chlorate after another cell run as the Chlorate concentration will be higher.
Another good way to get Sodium Chlorate out of the solution it to add some concentrated NaCl to the solution. This is called 'salting out' in the industry. The solubility of the Chlorate decreases markedly as the Chloride concentration goes up. The NaCl will be used when you start running the cell again. Take a note of the amount of Chloride that you added. You can lower the temperature of your electrolyte to below Zero to get out a bigger crop of Chlorate . Salting out is described below using information from Encylcopedia of Chemical Processing and Design (see Google books).
The mutual solubility diagram below is in units of Grams of solutes per 100ml water.
| Solid | Cell liquor of compositon A | Mother Liquor | Crystallization by adding Salt | NaClO3 crystalized | Mother liquor | Crystallization by water evaporation | NaClO3 crystalized |
| NaClO3 | 82 | 68 | - | 14 | 45 | - | 37 |
| NaCl | 14.5 | 22 | 7.5 (added) | - | 14.5 | - | - |
| H2O | 100 | 100 | - | - | 66 | 34 (evaporated) | - |
Note that Aluminium containers are not suitable for boiling the Chlorate solution in, as it will blacken the produce with corrosion products.
The solid Chlorate can be washed with very cold water and put into the Perchlorate cell.
When your chlorate cell is stopped running it will have a quantity of Hypochlorite (an intermediate product) in it. It is recommended that this hypochlorite be destroyed before going on to recover your solid Na Chlorate or make K Chlorate. This can be done by boiling your solution for about 15 minutes with the pH at neutral (you may have to do this anyways to concentrate the solution if you are separating out solid Sodium Chlorate) or by adding about 1g urea per litre of solution (boil). Hydrogen Peroxide will also destroy Hypochlorite as will Sodium Sulphite and Ammonia.
It is difficult to get rid of the Hypochlorite by boiling alone. I don't know what are the disadvantages of Hypochlorite in the Chlorate. It won't matter very much if you are going on to make Perchlorate as it will eventually all be destroyed in the Perchlorate cell.
The cell liquor (Mother liquor as it is called in industry) will now be returned to the Chlorate cell. Dry the extracted Chlorate and weigh, this will give you an idea of what quantity of Chloride and Chlorate are in the liquid.
It should be noted that Sodium Chlorate is very soluble and you may be disappointed with the yield of Sodium Chlorate that is obtained when you take product from your cells for the very first time.
There will be large amounts of Sodium Chlorate still dissolved in the mother liquor and when this is recycled into the cells you will get a much bigger yield of Sodium Chlorate in subsequent runs of the cell. If you have not been topping up your cells with Sodium Chloride when they were running for the very first time you will not be able to get any Chlorate to come out of solution by cooling alone, you will have to boil off some water in order to get Chlorate to ppt out. In subsequent extractions (assuming you recycled your mother liquor into the cells) the Sodium Chlorate concentration will be much higher and more will ppt out. You may still wish to boil off some water as described above.

I do not know how to remove Persulphates or Flourides from the electyrolyte nor do I know what effect small amounts of Persulphates or Flourides will have on pyrotechnic compositions.
HIT THE BACK BUTTON ON YOUR WEB BROWSER