Species and Genus Level Evolution in the Fossil Record
A fundamental prediction of evolution is that new species originate from preexisting species, that is, by speciation. Speciation may be cladogenetic, where one species diverges into two species, or anagenetic, in which change occurs within a single lineage, without branching, such that the two end member forms are recognized as seperate species. Although speciation has been demonstrated to occur in the modern world, paleontologists must use fossil evidence to reconstruct past speciation events and patterns of divergence during the past. The completeness with which any speciation 'event' can be documented by fossil evidence is a complex function of various factors, including paleobiogeography, preservation, human sampling, and evolutionary tempo itself. Population size and geographic distribution of species, the nature of sedimentation and the geologic record, the effects of taphonomy, paleontological sampling, and of course, the mode, tempo duration of speciation, all constrain the resolution of the fossil evidence. Obviously, a morpho-transition occurring rapidly in a geographically isolated population of soft-bodied organisms inhabiting environments with low preservation potential will not be expected to leave a detailed fossil record. Like all inferences about past events, inferences about speciation from the fossil record will always be limited to a greater or lesser degree by incomplete knowledge.
Nevertheless, as Benton and Pearson (2001) point out, "some parts of the fossil record are astonishingly complete and well-documented, and patterns of lineage-splitting can be examined in detail" (p. 405). One group of organisms for which we could expect to find a comparatively complete record of speciation and morphological change over time are the tiny, shelled marine protists such as foraminifera, radiolaria and coccoliths. . The study of these groups has been facilitated by the retrieval and study of numerous oceanic sediment cores by the Ocean Drilling Project and the Deep Sea Drilling Project over the past 50 years or so. Since many of these groups have wide geographic distributions, live in environments where conditions for preservation are favorable, and large numbers of them can be easily extracted and identified from deep-sea sediment cores, speciation in these groups can be documented at a level of detail unprecedented for other fossil groups. [For the same reason these groups make excellent index fossils for worldwide straigraphic correlation. Indeed, much of Phanerozoic time can be reliably subdivided on the first and last appearances of diverse microfossil taxa, and this biostratigraphic timescale has been independently tested and greatly enhanced by combination with paleomagnetic and isotopic data (e.g. Berggren et al., 1995a,b).] Referring to Darwin's view of the incompleteness of the fossil record, micropaleontologist Paul Pearson (1998) writes:
Unknown to Darwin, uninterrupted sedimentation does occur in the open ocean, especially on aseismic ridges and plateaux. These areas experience a continuous rain of particles to the sea bed, and are among the most geologically quiescent places on Earth. A steady build-up of sediment is the result. . . . The sediments in question are composed mainly of the shells of microscopic plankton such as foraminifera, radiolaria, diatoms and coccolithophorids. Large numbers of individuals can easily be extracted. Their evolution can be followed through geological time, simply by comparing one closely spaced sample with the next. This reveals morphologically isolated and continuous lineages which it is reasonable to infer represent lines of genetic descent. These lineages sometimes split from one another, and often evolve gradually over vast periods of time, or become extinct. . . Does the fossil record provide a true and accurate record of first and last occurrences of species? Emphatically, the answer is yes! Microfossils are used routinely for biostratigraphic correlation by thousands of specialists the world over. This would not be possible unless the sediment record was good and reliable. We now know (within fairly precise limits) when hundreds of species of mineralizing plankton arose and became extinct, through a history that spans over a hundred million years.
Well-documented examples of speciation and gradual morphological change in marine microfossils (e.g. forams, radiolarians, diatoms, and coccoliths) are increasingly common, particularly from the Cenozoic but also from earlier periods. For instance, Wei and Kennet (1988) provide evidence for the origin of the foram species Globoconella sphericomiozea from G. conomiozea terminalis about 5ma based on fossil material from sediment cores from four Deep Sea Drilling Project sites (DSDP 284, 207A, 208, and 588). Motoyama (1997) provides evidence for the Pliocene (~2.5ma) origin the radiolarian species Cycladophora davisiana from C. sakaii, and its subsequent morphologic evolution, based on fossil material from DSDP core 192. Sorhannus et al. (1998, 1999) provide evidence for the divergence of the diatom Rhizosolenia praebergonii and and R. sigmoida from R. bergonni at about 3ma, based on fossil records from several Pacific cores. Lazarus et al. (1985) and Lazarus (1986) provide evidence for the origin of the radiolarian species Pterocanium prismatium from P. charybdeum around 4ma, based on fossil material from sediment cores from the northwest Pacific. Kuwahara (1997) document evolutionary changes in the radiolarian genus Albaillella from Japanese chert sections. Raffi et al. (1998) provides evidence for the Miocene origin of several species of nannofossils from western Atlantic sediment cores, ODP Leg 154. They write: "Examination of Middle-Late Miocene sediments recovered during ODP Leg 154 in western equatorial Atlantic has led to identification of evolutionary transitions in some groups of late Neogene calcareous nannofossils. Through analyses of high resolution samples (10-cm sample interval equivalent to average interval of 6 kyr) we were able to document the origin of the genera Catinaster, Amaurolithus, and Ceratolithus, and the nannofossil species Discoaster berggrenii and D. quinqueramus. The presence of intermediate morphotypes between end-members representing distinct species sheds new light on phylogenetic relationships and/or confirms relationships suggested in previous studies. . . Successive branching from Triquetrorhabdulus rugosus is demonstrated for Amaurolithus primus, `Amaurolithus amplificus', and Ceratolithus acutus" (p. 17).
An Example: The Evolution of Orbulina
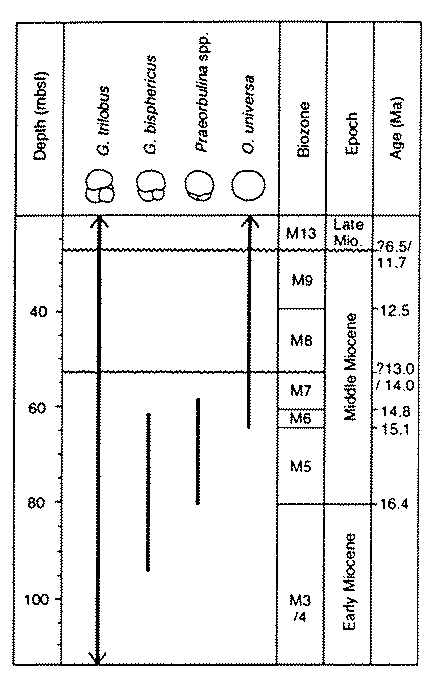
Pearson et al (1997) present fossil evidence for the evolution of the extant tropic-subtropic planktonic foraminifer Orbulina universa from a Miocene sediment core from Limalok Guyot in the tropical Pacific (Ocean Drilling Program Site 871).
"Orbulina is one of the most striking members of the mineralized plankton by virtue of its spherical shell. When the sphere is broken, a small, 'Globigerinoides-like' trochospiral shell can be found attached to the inner wall of the sphere (e.g. Vilks and Walker 1974). Thus, the sphere of Orbulina is in fact a greatly enlarged chamber which completely engulfs the earlier-formed part of the foraminifera test" (p. 296).
They note that the evolutionary transition from Globigerinoides to Orbulina, which occurs between ~16-15Ma, has been described from "all the major tropical ocean basins," and that "comparison with geomagnetic polarity reversals has shown that the various stages in the evolutionary transition occurred approximately simultaneously in the tropical Pacific, Indian, and Atlantic Oceans" (p. 296. See Berggren et al., 1995). Pearson et al. use stable carbon and oxygen isotope analyses to assess whether the speciation event was allopatric (for instance, population isolation as the result of depth-partioning, as proposed by Haynes 1981), or sympatric. They show that the isotopic offsets expected expected from depth-partitioning are not observed, and thus speciation must have occurred sympatrically. A slight offset develops between O. universa and G. trilobus only ~2Ma later in the Miocene, indicating that depth-partitioning amongst these forams developed after speciation. They conclude:
The evolutionary stages which result in Orbulina universa can be identified the world over, and indeed provide a series of useful biostratigraphical datums for global correlation. There is no evidence for peripheral isolation and recolonization. Indeed, this would seem unlikely, given the biology of the group and the nature of the their environment. This isotopic study has show that the evolution of Orbulina took placewithout any discernible change in depth habit or symbiotic association. It may therefore be taken as a good example of anagenesis in place and sympatric speciation (p. 310).

Image linked from Don Lindsay's Orbulina webpage. Originally from Pearson et al., 1997.
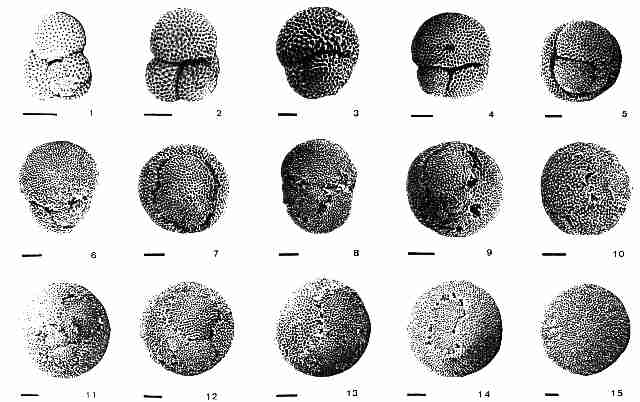
Image linked from Don
Lindsay's Orbulina webpage. Originally from Pearson et al.,
1997.
| From Pearson et al., 1997, p. 297: "Planktonic foraminifera from ODP site 871, Limalok Guyot, illustrating the Globigerinoides-Orbulina transition. 1. Globigerinoides trilobus, spiral side, showing supplementary aperature. . . 2. G. trilobus, umbilical side. The primary aperature is hidden in the central umbilical depression. . . 3. G. bisphericus, showing enlarged final chamber. . . 4, 5. Praeorbulina sicana (two views of same specimen). Four small aperatures are present in the suture around the base of the final chamber. . . 6. Another specimen of Praeorbulina sicana . . . 7. Praeorbulina curva. Note spherical morphology and multiple slit-like aperatures. . . 8. Praeorbulina globerosa, showing bispherical morphology . . . 9, 10. Praeorbulina glomerosa- circularis transitional specimens. . . 11-14. Praeorbulina circularis, showing variation in the proportion of the test occupied by the final chamber. . . 15. The end-form of the lineage, Orbulina universa, with entire sphere . . . scale bars are 100 Ķm." |
Another Example: The Prolecanitid Ammonoids
Distribution of Paleozoic Prolecanitid genera. From Saunders and Work (1997), based on biostratigraphic data from Russia, Germany, Canada, the USA, England, and elsewhere. Ruzhencev and Bogoslovskaya 1971 , 1978 ; Weyer 1972 ; Nassichuk 1975 ; Manger and Saunders 1982 ; Becker 1993 ; Boardman et al. 1994 ; Bogoslovskaya et al. 1995 ; Riley 1996 ; Glenister, Furnish, and Zhou unpublished.
Saunders and Work (1997) quantify change in Prolecanitid shell morphology over time using 21 different parameters, including coiling geometry (the W-D-S parameters of Raup 1967 [W = whorl expansion rate, D = degree of umbilication, S = shape of whorl]), aperature shape, and ornamentation (ribbing, nodes, grooves). These morphological features show slow and gradual changes throughout the range of the Prolecanitidae. These changes include gradual decreases in D (60%) and S (16%) parameters, and a slow increase (+25%) in the W parameter. Saunders and Work (1997) note that "such slow and gradual changes over such a long time span are known in no other ammonoid lineage."
Saunders and Work (1997) also compute Suture Complexity Indices (SCI) for each genus of Prolecanitids. The suture morphology in this group changed dramatically over time, from very simple sutures in the earliest [Devonian] Prolecanitids to extremely complex-sutured Prolecanitids in the late Paleozoic. Mean suture complexity "increased more than eight-fold (from a mean SCI of 7 to 58) over the 108 m.y. prolecanitid range" (p. 306). This increase in complexity of the sutures is a result of iterative processes of addition of umbilical lobes, serration of lobes, and the subdivision of lateral and ventral lobes. Saunders and Work (1997) summarize the evolution of the Prolecanitidae:
"As shown here, the Prolecanitida evolved extreme sutural complexity independently of their relatively static shell morphology. Sutural evolution involved adding as many as 12–15 replicate, U-shaped umbilical lobes, originating at the umbilicus and migrating across the flanks during both ontogeny and phylogeny. In addition, a number of different distinctive patterns of increasing complexity were utilized (see above). These, combined with consistent trends in conch form and ornament, have permitted reconstruction of widely accepted taxonomic and phylogenetic schemes (e.g., Ruzhencev 1949 ; Weyer 1972 ; Nassichuk 1975 ; Leonova 1989 ; Glenister, Furnish, and Zhou unpublished). . .
The ancestral family Prolecanitidae (Tournaisian–Namurian) shows an increase from two (Protocanites) to five (Dombarocanites ) umbilically derived lobes on the flanks. These were widely umbilicate smooth-shelled forms (e.g., Protocanites) in which the ventral lobe remained simple and undivided. The Prolecanitidae became extinct in the Namurian, shortly after giving rise to the Daraelitidae in the late Visťan.
In the descendant Daraelitidae (Visťan–Wordian) the number of umbilical lobes increased, the trifid ventral lobe became proportionately wider, and both the ventral and adjacent lateral lobes developed fine serrations (e.g., Boesites). The Daraelitidae extended into the Permian, providing the rootstock for Permian representatives of the ceratitid superfamily Xenodiscaceae, distinguished initially by subtle changes in the ventral lobe (bifid, rather than trifid as in the daraelitids).
In the ancestral medlicottiid family Pronoritidae (Visťan–Wordian) the originally broad, bifid ventrolateral lobe (e.g., Pseudopronorites) was increasingly subdivided, while the ventrolateral saddle of the first lateral lobe was progressively corrugated (e.g., Uddenoceras, Artinskia, Eumedlicottia, ) in the Medlicottiidae (Desmoinesian–Triassic). The Pronoritidae are characterized by involute compressed conchs (e.g., Pseudopronorites), which are further distinguished in the descendant Medlicottiidae by the presence of a row of prominent nodes near each ventrolateral shoulder (e.g., Artinskia; Eumedlicottia). The Medlicottiidae expanded gradually through the Missourian–Sakmarian (2–6 genera per stage), and following spectacular diversification in the Artinskian (acme of the Prolecanitida: 13 genera), progressively dwindled toward extinction as a single genus (Episageceras) in the Lower Triassic.

Plots of mean suture complexity (SCI) in Prolecanitida over time (Saunders and Work, 1997). illustrating the eight-fold overall increase in mean SCI over the 108 m.y. range of the group. From bottom to top, sutures are illustrated for Protocanites (SCI=6); Boesites (SCI+13), Pseudopronorites (SCI=29), Neouddenites (SCI=40), Artinskia (SCI=65), and Bamyaniceras (SCI=76).
References
Benton, M.J., and Pearson, P.N., 2001. Speciation in the fossil record. Trends in Ecology and Evolution 16, pp. 405-411.
Berggren, W.A., Kent, D.V., Swisher, C.C., III, and Aubry, M.P., 1995a, A revised Cenozoic geochronology and chronostratigraphy, in Berggren, W.A., Kent, D.V., Aubrey, M.-P., and Hardenbol, J., eds., Geochronology, Time Scales and Global Stratigraphic Correlation: SEPM (Society for Sedimentary Geology) Special Publication No. 54, p. 129-212.
Berggren, W. A., Hilgen, F.V., Langereis, C.G., Kent, D.V., Obradovich, J.D., Isabella Raffi, Raymo, M.E., and Shackleton, N.J., 1995. Late Neogene chronology: New perspectives in high-resolution stratigraphy. GSA Bulletin: 107, pp. 1272–1287.
Kucera, M., and Malmgren, B.A., 1998. Differences between evolution of mean form and evolution of new morphotypes: an example from Late Cretaceous planktonic foraminifera. Paleobiology 24, pp. 49-63.
Kuwahara, K., 1997. Evolutionary patterns of Late Permian Albaillella (Radiolaria) as seen in bedded chert sections in the Mino Belt, Japan, Marine Micropaleontology 30, pp. 65-78.
Lazarus, D.B., R.P. Scherer, and D.R. Prothero., 1985. Evolution of the radiolarian species-complex Pterocanium: a preliminary survey. Journal of Paleontology 59, pp. 183-221.
Malmgren, B.A., and Kennett, J.P., 1981. Phyletic gradualism in a Late Cenozoic planktonic foraminiferal lineage, DSDP Site 284, southwest Pacific. Paleobiology 7, pp. 230-240.
Motoyama, I., 1997. Origin and evolution of Cycladophora davisiana Ehrenberg (Radiolaria) in DSDP Site 192, Northwest Pacific, Marine Micropaleontology 30, pp. pp. 45-63.
Pearson, P.N., Shackleton, N.J., and Hall, M.A., 1997. Stable isotopic evidence for the sympatric divergence of Globigerinoides trilobus and Orbulina universa (planktonic foraminifera), Journal of the Geological Society, London 154, pp. 295-302.
Pearson, P.N., 1998. The glorious fossil record. Nature online, November 19: http://www.nature.com/nature/debates/fossil/fossil_1.html
Raffi, I., Backman, J., and Rio, D., 1998. Evolutionary trends of tropical calcareous nannofossils in the late Neogene, Marine Micropaleontology 35, pp. 17-41.
Sorhannus, U., 1990. Tempo and model of morphological evolution in two Neogene diatom lineages. Evolutionary Biology, 24, pp. 329-370.
Sorhannus, U., et al., 1999. Iterative evolution in\par the diatom genus Rhizosolenia Ehrenberg. Lethaia 24, pp. 39–44.
Some Abstracts:
Raffi, I., Backman, J., Rio, D., 1998. Evolutionary trends of tropical calcareous nannofossils in the late Neogene, Marine Micropaleontology 35, pp. 17-41.
Examination of Middle-Late Miocene sediments recovered during ODP Leg 154 in western equatorial Atlantic has led to identification of evolutionary transitions in some groups of late Neogene calcareous nannofossils. Through analyses of high resolution samples (10-cm sample interval equivalent to average interval of 6 kyr) we were able to document the origin of the genera Catinaster, Amaurolithus, and Ceratolithus, and the nannofossil species Discoaster berggrenii and D. quinqueramus. The presence of intermediate morphotypes between end-members representing distinct species sheds new light on phylogenetic relationships and/or confirms relationships suggested in previous studies. The mode and timing of the evolutionary transitions described are discussed. Successive branching from Triquetrorhabdulus rugosus is demonstrated for Amaurolithus primus, `Amaurolithus amplificus', and Ceratolithus acutus. A new genus is therefore established, Nicklithus, type species Nicklithus amplificus n. comb. A new species is described, Ceratolithus larrymayeri n. sp. The genera Triquetrorhabdulus, Amaurolithus, Nicklithus and Ceratolithus all belong to the family Ceratolithaceae.
Kucera, M., and Malmgren, B.A., 1998. Differences between evolution of mean form and evolution of new morphotypes: an example from Late Cretaceous planktonic foraminifera. Paleobiology 24, pp. 49-63.
Morphological evolution in the Late Cretaceous (Maastrichtian) Contusotruncana lineage of planktonic foraminifera was studied at DSDP Sites 525 (South Atlantic) and 384 (North Atlantic). A multivariable approach was used to separate aspects of form controlled by geographical variation (size, spiral roundness of the test, percentage of kummerform specimens) from those due to changes that occurred simultaneously in geographically distant populations of the lineage (shell conicity, number of chambers in the last whorl).
A gradual increase in mean shell conicity was observed over the last 3 million years of the Cretaceous. It arose from the combination of a rapid development of highly conical shells after 68.5 Ma and a long-term trend of progressive disappearance of the ancestral morphotype. Therefore, despite the gradual change in “mean form,” the morphological evolution in the Contusotruncana lineage differs from the classical image of phyletic gradualism. The gradual increase in mean shell conicity in the lineage was accompanied by a remarkable decrease in its absolute abundance (shell accumulation rate), suggesting that the changes in shell morphology might not have been neutral with respect to natural selection. Apparently, gradual change in “mean form” of fossil lineages does not require an equally gradual development of morphological novelties. It may be caused by natural selection operating on a constant range of variation in populations living in environments without geographical barriers.
Kuwahara, K., 1997. Evolutionary patterns of Late Permian Albaillella (Radiolaria) as seen in bedded chert sections in the Mino Belt, Japan, Marine Micropaleontology 30, pp. 65-78.
The stratigraphic distribution of Late Permian Albaillella (Radiolaria) was studied in bedded chert sections from the Mino Belt, central Japan. There is a change in relative frequency up section, where the highest frequency of occurrence changes from Albaillella triangularis in the lower part of the sections to the A. excelsa-A. lauta Lineage Group (A. excelsa, A. sp. B, A. sp. A, A. flexa and A. lauta), then to A. levis and A. sp. aff. A. levis in the uppermost parts. The part of highest frequency is interpreted to represent each of their evolutionary acmes.
A morphometric study was applied to the A. excelsa-A. lauta Lineage group where the length from proximal part of wing to aperture was measured. A gradual morphological change of this feature through time is seen in the A. excelsa-A. lauta Lineage group.
The phylogenic relationships of Late Permian Albaillella are constructed on the basis of this morphological change as observed in stratigraphic succession. An evolutional lineage for A. triangularis-A. excelsa-A. sp. B-A. sp. A-A. flexa-A. lauta is presented.
Lazarus, D.B., 1986. Tempo and mode of morphologic evolution near the origin of the radiolarian lineage Pterocanium prismatium. Paleobiology 12, pp. 175-189.
Pterocanium prismatium is a fossil radiolarian lineage commonly found in equatorial sediment cores throughout the world ocean. It first appeared in the fossil record ~4ma, and became extinct 1.8ma. Previous examination of phylogenetic pattern in late Neogene Pterocanium (Lazarus et al. 1985) observed the origin of P. prismatium from P. charybdeum in severak sediment cores from the equatorial Pacific and Indian Oceans. The P. charybdeum lineage is extant today in tropical and subtropical waters, and extends back in time well into the Miocene. The origin of P. prismantium from P. charybdeum thus represents biological speciation, as it involved the splitting of one ancestral lineages into two descendent ones (p. 175).
Morphometric examination of cladogenesis and phyletic evolution in two late Neogene sister lineages of marine microfossils (Pterocanium prismatium and Pterocanium charybdeum, Radiolaria) from two equatorial Pacific sediment cores was undertaken to better understand the rate of cladogenesis and its relation to subsequent phyletic change. The origin of P. prismatium from P. charybdeum ~4ma ago has been estimated to take place over an interval of ~500,000 yr. Results show that the speciation event consists of two distinct phases. The firsst phase, cladogenesis, occurred relatively rapidly (on the order of 50,000 yr). A second phase of relatively rapid divergent phyletic evolution away from the common ancestral state followed in both descendent branches and continued for at least 500,000 yr after completion of the cladogenetic event. Net evolutionary rates over the next 2ma appear to be much slower. Individual characters change by as much as 2 population standard deviations during cladogenesis, and by a total of approximately 3 standard deviations over 2.5ma of phyletic evolution. Up to 5 population standard deviations of change during <-50,000 yr of cladogenesis, and 7 additional standard deviations of phyletic change over 500,000 are observed in multivariate (discriminant function) indices of morphologic difference. The measured pattern does not appear to be either strictly 'punctuated' or strictly 'gradual,' but instead shows features of both hypothesis (p. 175).
Motoyama, I., 1997. Origin and evolution of Cycladophora davisiana Ehrenberg (Radiolaria) in DSDP Site 192, Northwest Pacific, Marine Micropaleontology 30, pp. pp. 45-63.
This paper documents the evolutionary history of Cycladophora davisiana Ehrenberg from an uppermost Miocene to Pleistocene sedimentary record in the high-latitude Northwest Pacific. It apparently evolved from C. sakaii Motoyama through a series of intermediates. C. sakaii has a relatively large shell with an external spongy layer. The evolutionary transition is characterized by a relatively rapid decrease in thorax size with a reduction of the spongy appendage. This change occurred during about 0.4 m.y. from 2.8 to 2.4 Ma without cladogenesis. Following this interval, a decrease in thorax size continued gradually up to the Recent, resulting in very small morphology. Although the population of C. davisiana first appeared at about 2.5 Ma, some morphotypic specimens may occur in earlier periods as indistinguishable very small endmembers in the C. sakaii populations.
"Substantial geographic coverage in paleontological study is essential in testing evolutionary models of phyletic gradualism and punctuated equilibrium. We present a multivariate morphometric study of the late Neogene planktonic foraminiferal clade Globoconella using specimens from four Deep Sea Drilling Project sites (DSDP 284, 207A, 208, and 588) along a latitudinal traverse in the southwest Pacific" (p. 345).
"The gradual transformation of G. (G.) conomiozea terminalis (a form retaining a keel) into G. (G.) sphericomiozea (a form lacking a keel) occurred during an interval of about 0.2m.y., with all measured morphological variables showing continuous and steady changes. The evolution of the central populations follows the model of phyletic gradualism. In peripheral populations, the origin of the descendent species G. (G.) pliozea from the ancestor G. (G.) conomiozea terminalis occurred very rapidly within an interval of less than 0.01m.y. . . The evolution os the Globoconella clade shows both phyletic gradualism and puncuated equilibrium. These two 'alternative' evolutionary models complement each other rather than being mutually exclusive. Both models are indespensible towards providing a complete picture of the evolution of Globoconella" (p. 345).
"During the interval of geographic isolation, central populations gradually evolved into a new chronospecies, G. sphericomiozea. It took about 0.17m.y. (from 5.14 to 4.97Ma) to transform the whole ancestral population of the highly conical, keeled morphocline (G. conomiozea terminalis) into the descendent subglobular, non-keeled species, G. sphericomiozea. The evolution in the central populations follows the model of phyletic gradualism. In contrast, the peripheral populations rapidly gave rise to a new species, G. pliozea, a form resembling flattened members of the ancestral populations. The origination of G. pliozea is inferred to be an allopatric speciation occurring within an interval of less than 0.01m.y. Following speciation, the new species remained in morphological stasis for about 0.6m.y. . . The evolution of G.pliozea follows the model of punctuated equilibrium" (p. 361).