
Worksheet 13.1, 13.2, 14.1, 15.1 Electron Configurations
Read “The Evolution of Atomic Models” in your book p. 361-364.
1. Sketch and describe the evolution of the atomic model from the Thomson model to the Quantum mechanical model. Write a more in-depth description than just the caption of the picture in the text.
a) Thomson model
b) Rutherford model
c) Bohr Model
d) Quantum mechanical model
Read “Atomic Orbitals” p. 364-366.
2. In the quantum mechanical model
a) what does each principal quantum number refer to?
b) how does the number of sublevels in an energy level compare to the principal quantum number?
c) are electrons confined to a fixed circular path? If not, where are the electrons found?
d) what shape(s) are s, p, and d orbitals?
e) what do the nodes in p and d orbitals represent?
3. Fill in the following chart:

Read “Electron Configurations” p. 367-370.
4. Look at the attached “Aufbau Diagram for electron configurations” and the “Example - electron configuration for iodine”.
a) Notice that iodine has electrons in 5 principle energy levels. In what period is iodine found on the periodic table?
b) Notice that iodine has 7 electrons in the last principle energy level (2 in the 5s and 5 in the 5p). In what number group A is iodine found on the periodic table?
5. Following the example for iodine, fill in the electrons for arsenic on the blank Aufbau diagram. Write arsenic’s electron configuration below:
Electron Configurations and the Periodic Table
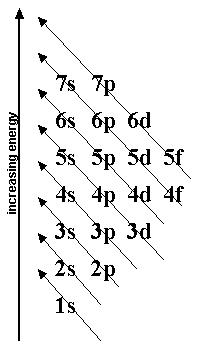
6. Write the electron configurations for the period 2, 3, and 4 noble gases. You may find it easier to use the smaller version of the Aufbau diagram above.
Group 8A or 0
a) period 2 - Ne
b) period 3 - Ar
c) period 4 - Kr
7. Write the electron configurations for the period 2, 3, and 4 alkali metals.
Group 1A
a) period 2 - Li
b) period 3 - Na
c) period 4 - K
8. Write the electron configurations for the period 2, 3, and 4 halogens.
Group 7A
a) period 2 - F
b) period 3 - Cl
c) period 4 - Br
9. What relationship can you see between an element’s electron configuration and its period?
How many principle energy levels would you expect the electrons of aluminum to occupy? Why?
10. What relationship can you see between an element’s electron configuration and its group A number?
How many total electrons would you expect to find in the outermost principle energy level of nitrogen? Why?
Read “Classifying Elements by Electron Configuration” p. 391-396.
11. How are these elements classified into groups according to their electron configurations?
a) noble gases
b) representative elements
c) transition metals
d) inner transition metals
12. Why are there some exceptions to the electron configurations for elements as given by the Aufbau diagram? (see p. 370)
13. Noble gases are sometimes called the inert gases because they do not participate in many chemical reactions. They have filled outermost s and p orbitals. How many electrons in this outer principle energy level?
Read “Electron Configurations for Anions and Cations” p. 414-418.
14. In 1916, chemist Gilbert Lewis developed the octet rule to explain why atoms form certain kinds of ions and molecules. The octet rule says that in forming compounds, atoms tend to achieve the electron configuration of a noble gas. Looking at your answer to #13, why do you think this is called the octet rule?
15. Look at your electron configurations for the Group 1A alkali metals.
a) Explain why, according to the octet rule, alkali metals tend to lose an electron and form +1 ions.
b) Write the electron configuration of Na+.
16. Look at your electron configurations for the Group 7A halogens.
a) Explain why, according to the octet rule, halogens tend to gain an electron and form –1 ions.
b) Write the electron configuration of F–.
17. Predict what charge ions in Group 2A alkaline earth metals would form. Why do you think this is so?
18. Predict what charge ions in Group 6a nonmetals would form. Why do you think this is so?
19. Write the electron configurations for
a) H+
b) Mg2+
c) O2–
d) P3–
20. Give a the chemical symbol of a neutral atom and two ions that have the following electron configurations:
a) 1s22s22p63s23p6
a) 1s22s22p63s23p63d104s24p6

