Lab - Reactivity Trends in the Periodic
Table
Purpose: To investigate reactivity trends down groups in nonmetals and metals and across periods.
Part A: Reactivity of the
Halogens
Materials:
mineral oil
chlorine water
iodine solution
KCl solution (1 M)
KI solution (1 M)
24-cell plastic wellplate
5 pipettes
toothpicks
Instructions for making solutions (this may already have been done by your
teacher):
chlorine water (100 ml Chlorox with 6 drops HCl)
iodine solution (1 g KI + 0.5 g I2 – dilute to 50 ml)
1 M KCl (4 g KCl in 50 ml water)
1 M KI (8 g KCl in 50 ml water)
Procedure:
- Obtain one pipette each of mineral oil, chlorine water, iodine solution, KCl solution and KI solution.
- Record the colors of each of the four solutions in Data Table 1.
- Add six drops of mineral oil to wells 1-6.
- Add 6 drops of chlorine water to wells 1 and 5
- Add 6 drop of iodine water to wells 2 and 6
- Add 6 drops of KCl to wells 3 and 6.
- Add 6 drops of KI to wells 4 and 5.
- Stir each well vigorously with a CLEAN toothpick for each.
- Let the layers settle.
- Examine and record the colors of the aqueous layer and mineral layer for each well in Data Table 2.
- Dispose of materials as outlined by your teacher. Wash and dry the wellplate.
Data:
Table 1
|
|
name |
chemical formula |
state at room temp. |
color of aqueous solution (before
adding to wells) |
|
halogen |
chlorine |
Cl2 |
gas |
|
|
halogen |
iodine |
I2 |
solid |
|
|
halide salt |
potassium chloride |
KCl |
solid |
|
|
halide salt |
potassium iodide |
KI |
solid |
|
Table 2
|
well # |
contents |
color |
|
|
|
|
aqueous layer (bottom) |
mineral oil layer (top) |
|
1 |
chlorine water |
|
|
|
2 |
iodine solution |
|
|
|
3 |
aqueous KCl |
|
|
|
4 |
aqueous KI |
|
|
|
5 |
chlorine water + KI |
|
|
|
6 |
iodine solution + KCl |
|
|
Data Analysis
1) Looking at your results for well #’s 1-4, do the following dissolve better in water or mineral oil?
a) halogens? ______________________
b) halide salts? ______________________
2)
To
compare the reactivity of chlorine and iodine with each other, the halogen of
each is mixed with the halide of the other (see well #’s 5 and 6). For example,
in well #5, chlorine water has been
mixed with KI. There are two possibilities:
(i) There is a
reaction: Cl2 + 2KIà 2KCl + I2
or
(ii) There is no reaction: Cl2 + 2KI
à Cl2 + 2 KI
a)
If
possibility (i) happens, in which layer (mineral oil
or water) would you expect to find the I2? Explain.
b)
If
possibility (ii) happens, what would you expect to see? Be specific.
c)
According
to your results, did a reaction occur in well #5? Explain.
3)
In well #6, iodine water has been mixed with KCl. There are two possibilities:
(i) There is a
reaction: I2 + 2KClà 2KI + Cl2
or
(ii) There is no reaction: I2 + 2KCl
à I2 + 2KCl
a)
If
possibility (i) happens, what would you expect to see
in the mineral oil layer? Explain.
b)
If
possibility (ii) happens, what would you expect to see? Be specific.
c)
According
to your results, did a reaction occur in well #6? Explain.
4) If a reaction occurred in well #5, chlorine is more reactive than iodine, because chlorine replaced the iodine in the halide salt.
If a reaction occurred in well #6, iodine is more reactive than chlorine, because iodine replaced the chlorine in the halide salt.
Which is more reactive, chlorine or iodine? Explain.
5) Fill in the blanks using a periodic table and your results:
a) In what group # are the halogens? _____
b) What happens to the reactivity of nonmetals as you go down a group (increase or decrease)? _________ Explain how you know this from your results.
Part B: Reactivity of the
Alkaline Earth Metals
Materials:
1 cm strip of magnesium
small pellet of calcium
water
1 M hydrochloric acid (HCl)
24-cell plastic wellplate
2 pipettes
Procedure:
- Put the strip of magnesium in well #1.
- Put a small piece of calcium into well #3.
- Add 6 drops of water to wells #1 and #3.
- If a reaction occurs, a gas will be given off (it
will bubble). Rate of reaction can be determine by how rapidly it bubbles.
Record your observations in Data Table
- If the metal did not react, move it to the well underneath and add 6 drops of HCl.
- Record your observations in the data table.
- Dispose of materials as outlined by your teacher. Wash and dry the wellplate.
Data:
Table 3
|
element |
reacts in… (water, HCl,
neither) |
rate of reaction (slow, moderate, vigorous) |
|
magnesium |
|
|
|
calcium |
|
|
Data Analysis
- A metal that will react in water is more reactive
than one that will only react in hydrochloric acid. Which is more
reactive, Mg or Ca? Explain.
- Fill in the blanks using a periodic table and your results.
- In what group # are the alkaline earth metals? _____
- What happens to the reactivity of metals as you go down a group (increase or decrease)? Explain how you know this from your results.
Part C: Reactivity of Elements
Across a Period
Materials:
1 cm strip of magnesium
1 cm strip of aluminum wire
small amount of sulfur
water
1 M hydrochloric acid (HCl)
24-cell plastic wellplate
2 pipettes
Procedure:
- Put the strip of magnesium in well #1.
- Put the strip of aluminum into well #3.
- Put a small amount of sulfur in well #5.
- Add 6 drops of water to wells #1, 3 and 5.
- Record your observations in Data Table
- If the element did not react, move it to well underneath and add 6 drops of HCl.
- Record your observations in the data table.
- Dispose of materials as outlined by your teacher. Wash and dry the wellplate.
Data:
Table 3
|
element |
reacts in… (water, HCl,
neither) |
rate of reaction (slow, moderate, vigorous) |
|
magnesium |
|
|
|
aluminum |
|
|
|
sulfur |
|
|
Data Analysis
- List the elements (Mg, Al, S) from most to least reactive. Explain.
- Fill in the blanks using a periodic table and your results.
- In what period # are magnesium, aluminum and sulfur? _____
- What happens to the reactivity of elements as you go across a period (increase or decrease)? __________ Explain how you know this from your results.
Conclusions & Extensions:
- Using the results of the “lab” shown in Figure 1, circle ALL the correct answers.
- chlorine is more reactive than bromine / iodine / none of these
- bromine is more reactive than chlorine / iodine / none of these
- iodine is more reactive than chlorine / bromine / none of these
Explain your answers above.
- Go to http://www.chem.ox.ac.uk/vrchemistry/FilmStudio/alkalimetals/HTML/page01.htm and watch each of the movies showing reactions of alkali metal (group 1A).
- List the following in order from least to most reactive: K, Li, Na
- Explain your evidence for part a.
- Rubidium must be stored in oil and not exposed to the moisture in the air. why do you think this is so?
- Do your observations of the alkali metals agree with the trend for reactivity of metals that you found in part B? Why or why not?
- Which would you expect to be more reactive
- sodium or magnesium?
- beryllium or magnesium?
- bromine or fluorine?
You have investigated reactivity trends in the periodic table. There are other trends as well. Examine the specified figure for these trends.
- Figure 2. What is the trend in atomic mass as you go
- down groups?
- across periods?
- Figure 3. Examine the melting points.
- Which have higher melting points, metals or nonmetals?
- What is the trend in melting point for metals
i. across periods?
ii. down groups?
- What is the trend in melting point for nonmetals
i. across periods?
ii. down groups?
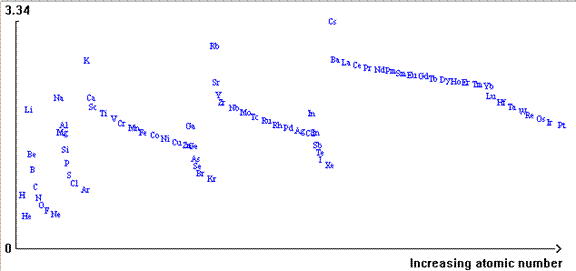
- In Figure 4, atomic radius is on the y-axis.
- Circle the chemical symbols of the alkali metals in Groups 1.What is the trend in atomic radius as you go down a group?
- Circle the chemical symbols of elements in Period 1.What is the trend in atomic radius as you go across a period?
Figure 1. – Reactivity of Halogens
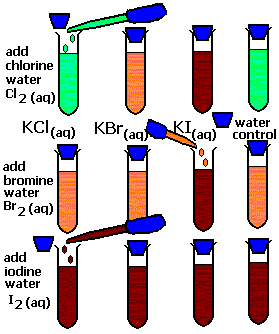
Figure 2. – Periodic table.
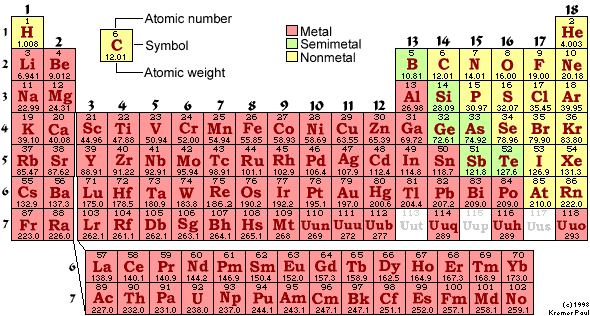
Figure 3. – Melting Points
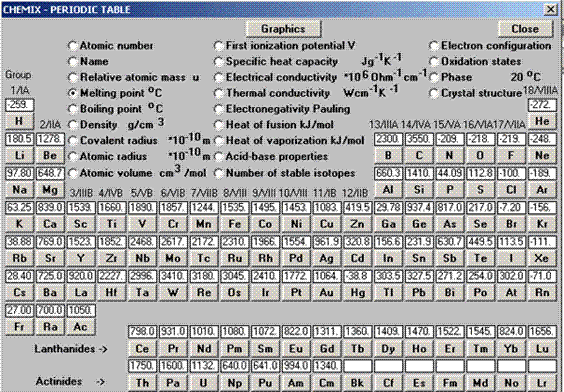
Figure 4 – Atomic radius vs Increasing Atomic
#