Unit 2 Study Guide
Atomic Structure & the Periodic
Table
E1. Trace the development of models of the atom to the present and describe
how each model reflects the scientific understanding of their time.
E6. Compare the physical and chemical characteristics of elements.
E7. Describe nuclear
reactions, including fusion, fission, and decay, their occurrences in nature,
and how they can be used by humans.
Activity #1 – Atomic
Structure
Read ATOMS AROUND US.
- Fill in the
blank: All elements are made of
______________.
- Label the
major subatomic particles in the atom on the diagram below.

- Fill in the
blanks: The ___________ and ____________are always in the center of the
atom. Scientists call the center of the atom the ________. The _________
are always found whizzing around the center in areas called __________.
- What do the
“0”, “-“, and “+” refer to on the diagram above?
Activity #2 – Atomic
Builder
The stuff you scrape off burnt
toast is made primarily of atoms of carbon. But what makes up a carbon atom --
or any other atom?
Here's a chance for you to
construct a carbon atom. You'll start with a hydrogen atom, which contains one
proton and one electron. Just add protons, neutrons, and electrons. By the way,
you must also build each proton and neutron from two types of quarks -- up
quarks and down quarks. Finally, some advice: try to keep the particles'
charges balanced. You'll have a difficult time if you don't. Before you start,
answer the questions below.
Read The Atom Builder
Guide to Elementary Particles.
- In activity
#1, you learned about the three major subatomic particles. It turns out that protons and neutrons
are made of even smaller particles.
What are these particles called?
- Record the
color code in the picture of an atom on this page (you will need it
later). What color represents the
- protons?
____________
- neutrons?
____________
- electrons?
____________
- Fill in the
“recipes” for the nucleons (particles found in the nucleus) below:
1 proton = ____ up quark(s) + ____ down
quark(s)
1 neutron = ____ up quark(s) + ____ down
quark(s)
Read The Atom Builder
Guide to Building a Stable Atom.
- Fill in the blanks: A stable atom has a net charge of
_______. In other words, it has an equal number of _________ and ________.
- When is an atom ionized? Why don’t you want this to happen in this activity?
- When does an atom become radioactive? Why don’t you want this to happen in
this activity?
- Up to how many electrons can occupy
- the 1st shell _______?
- the 2nd shell _______?
Now you are ready to build a
carbon atom! Open the Atom Builder.
- Construct a neutron from the up and down
quarks in the “Nucleon Assembly” area.
Move this to the hydrogen nucleus on the right. Did adding a neutron change hydrogen into helium?
- Construct
another neutron from the up and
down quarks in the “Nucleon Assembly” area. Move this to the nucleus on the
right. What happened to the atom?

- Construct a proton from the up and down quarks
in the “Nucleon Assembly” area.
Move this to the hydrogen nucleus on the right. Did adding a proton change hydrogen into helium? What else happened?
- Add an
electron to the atom. Try putting
it in the 2nd energy level (the outer circle). What happens? Where does the electron end up?
- At this
point you should have a stable helium
atom. How many electrons _____? Protons _____? Neutrons _____?
- Add another
electron to the atom. Try putting
it in the 1st energy level (the inner circle). Are you allowed to do this? Why or why not?
- Continue
building your atom in this manner until you have constructed a stable carbon atom. Show your carbon atom to your teacher
and have her initial below.
Teacher’s initials _____________
How many electrons
_____? Protons _____? Neutrons _____?
Activity #3 – Dream Journey into the Atom (The
Particle Picture)
You will need to use the
poster here. (You may get a print-out of this poster from
your teacher if you wish.) Matter is made of tiny particles. And those
particles are made of even tinier particles ... Name the particles
described in the clues. You will find all of the answers in the poster.
|
1 |
Brownian
motion: You see these microscopic specks of dust or smoke moving around ... |
|
|
|
|
|
|
|
|
|
|
2 |
...
because we believe they are pushed about by these particles of the air
... |
|
|
|
|
|
|
|
|
|
|
3 |
...
which are made up of these particles of oxygen and nitrogen. |
|
|
|
|
|
|
|
|
|
|
4 |
JJ
Thomson discovered these particles ... |
|
|
|
|
|
|
|
|
|
|
5 |
...
which orbit around this particle at the center of every atom ... |
|
|
|
|
|
|
|
|
|
|
6 |
...
which is made up of these positively-charged particles ... |
|
|
|
|
|
|
|
|
|
|
7 |
...
and these uncharged ones ... |
|
|
|
|
|
|
|
|
|
|
8 |
...
which are made up of these even tinier particles. |
|
|
|
|
|
|
|
|
|
Now,
use the 11 letters in the highlighted boxes to make a word which is the name of
a machine used for making sub-atomic particles move faster.
|
|
|
|
|
|
|
|
|
|
|
|
At the start of the twentieth century, there
was no way that scientists could hope to see individual atoms. Indeed, many
scientists still did not accept that matter was made of atoms.
Activity #4 – Dream Journey
into the Atom (Changing Pictures)
At the start of the twentieth century, there
was no way that scientists could hope to see individual atoms. Indeed, many
scientists still did not accept that matter was made of atoms. Since then, some very clever experiments have
allowed us to find out a lot more about the structure of atoms. Our picture of
the atom has changed a lot.
(You will find the answers to these
questions in the poster.)
1)
Model 1: The
‘pudding’ model
In the pudding
model:
a)
What is the dough? What charge does it have?
b)
What are the currants? What charge do they have?
c)
Why must there be equal amounts of positive and
negative charge?
2)
Model 2: The
‘nuclear’ model
In the nuclear
model:
a)
What is at the center of the atom? What charge does it
have?
b)
What particles orbit around the outside? What charge do
they have?
3)
Changing models
Ernest
Rutherford suggested an experiment to test the pudding model.
a)
What metal did he use as his target?
b)
Which particles did he fire at the target?
c)
What was the source of the particles?
d)
If the pudding model was correct, what would happen to
the particles?
e)
What was the surprising result of the experiment?
f)
How did Rutherford account for this result?
4)
Changing ideas
The word atom means indivisible.
a)
Is an atom indivisible?
b)
Why do you think we stick with the word atom?
Activity #5 – Dream Journey
into the Atom (Particles & People Puzzle)
Use the poster
to solve these clues:
|
1.
In 1897, I made a beam of electrons in a vacuum
tube. Who am I? |
|
|
2.
You’ll find me in an atom and in a lightning flash. I
sometimes travel along wires. I’m naturally negative! What am I? |
|
|
3.
It was a surprise to me when an alpha particle
bounced back! My prediction was completely
wrong! Who am I? |
|
|
4.
I may be small, but I’m a lot heavier than those
electrons. Maybe that’s why they orbit
around me! What am I? |
|
|
5.
I’m using electrons to study what is inside protons.
Who am I and where do I work? |
|
|
6.
I am one of these: a molecule, a proton, an electron,
an atom or a nucleus. I’m the only one
of these who isn’t made up of other particles. What am I? |
|
|
7.
Jude uses me in a beam with loads of others just like
me so that she can look into new materials.
What am I? |
|
|
8.
I work on the world’s biggest particle accelerator
looking for answers to big questions.
Who am I and where is the accelerator? |
|
|
9.
Gavin is working on me. I am going to be the new
version of the World Wide Web! What am
I? |
|
|
10.I use work done in particle
physics experiments to make better X-ray detectors which should help us to
treat cancer. Who am I? |
|
The next two of these have
answers but not clues! Can you think of
good clues for them?
|
11. |
Quark |
|
12. |
Molecule |
Activity #6 – Ions, Atomic
Number, Atomic Mass and Isotopes
- On this
page:

- Define atomic number.
- Click
“extra”. What defines the identity of an element?
- If you add
a proton to sodium, what element do you have? What is its atomic number?
- If you
subtract a proton from sodium, what element do you have? What is its atomic number?
- On this
page:
- define ion:
- define cation:
- define anion:


- If you
remove an electron from a neutral atom, does it become positive or
negative?
- If you add
an electron to a neutral atom, does it become positive or negative?
- On this
page:

- Define mass number.
- You can
figure out the number of neutrons in an atom of oxygen by subtracting its
_____________ (the number of protons) from its _____________.
- How many
neutrons are there in an oxygen atom with an atomic mass of 16? ________
- How many
neutrons are there in a sodium atom with an atomic mass of 23? ________
- On this
page:
- Define isotope.
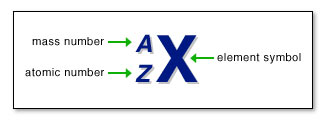
- The
standard format for writing an isotope is shown above, where A is the
__________ number ( __________ + __________ ) and Z is the __________
number (number of __________ )

- For a
neutral atom of boron-10, what is the
1. mass number _____
2. atomic number _____
3. number of protons _____
4. number of neutrons _____
- For
boron-11, what is the
1. mass number _____
2. atomic number _____
3. number of protons _____
4. number of neutrons _____
- On this
page:
- Define atomic mass.
- The units
for atomic mass are ____________________, or _____.
- One amu is defined as ____________ the mass of a
__________ atom (specifically the carbon-12 isotope). Therefore, the mass
of one atom of carbon-12 is exactly ________ __________.

- How do you
get the mass of boron (10.81 amu) that is found
on the periodic table? Do the calculation below:
1. boron-10 is found in nature 19% of the time
so
0.19 X 10.00 amu = ______
2. boron-11 is found in nature 81% of the time
so
3. 0.81 X 11.00 amu =
______
4. to get the average atomic mass of all the
isotopes of boron found in nature, add #1 and #2 above = ______
- Quiz
yourself: What element in the periodic
table
- has 23 protons? ________
- has an atomic mass of 14? ________
- has 12
electrons in a neutral atom? ________
- has 16 electrons in its -1 anion? ________
- has two
isotopes, one with a mass of 35 amu (77.5%
abundance) and one with a mass of
37 amu (22.5% of the time) ________
- Quiz
yourself:




Activity #7 – Electron Shells
Fill in the blanks using this website.
Electrons are arranged around the ______________
in ______________. For simplicity they can be thought of like mini-planets
orbiting a central sun, but it is closer to the truth to think of them as
"clouds" of electric charge around the Nucleus.

The shells are numbered ___________ from the
Nucleus. Fill in the maximum number of electrons found in each shell in the
table below.
|
Shell Number |
Maximum Number |
|
1 |
|
|
2 |
|
|
3 |
|
|
4 |
|
|
5 |
|
The Octet Rule:
In general, atoms are most stable when they have_________ electrons in their
outer-most shell. ( ______ means 8.) The exception is the ____________ shell
which is most stable with _________ electrons.
If you know the ___________ ___________ and ______________ ___________ of an element and the maximum number of electrons in each electron shell you can draw a diagram of the element.
For example: Sodium has an
Atomic Number of 11 and an Mass Number of 23.
This means an atom of Sodium has __________ Protons and therefore ________
electrons.
Since the number of Protons + Neutrons is __________
and there are _________ Protons there must be ________ Neutrons.
From the table above the electrons are arranged
as: First Shell = __________, Second Shell = _________, Third Shell = ________
(Giving a total of __________.)


Example 2: Chlorine has a Mass
Number of 35 and an Atomic Number of 17.
This means an atom of Chlorine has ___________
Protons and therefore _________ electrons.
Since the number of Protons + Neutrons is __________
and there are _________ Protons there must be __________ Neutrons.
From the table above the electrons are arranged
as: First Shell = __________, Second Shell =__________, Third Shell = __________
(Giving a total of _________.)
Activity #8 – A Periodic
Table of Colors
- Open this article.
Fill in the blanks
The periodic
table is organized like a big ________. The elements are placed in
specific places because of the way they _________ and ___________. If you have
ever looked at a grid, you know that there are __________ (left to right) and
_____________ (up and down). The periodic table has rows and columns, too, and
they each mean something different.


- Open The
Periodic Table of Colors.
Make a periodic table of colors by arranging them into groups and periods that have similar properties.
Note in the pictures above, that periods are horizontal rows and groups are vertical columns. In a periodic table, elements that are in the
same periods and groups have similar properties. A periodic table is also
arranged in a way that shows periodic or recurring trends in the properties of
elements.
Your periodic tables of colors should be
arranged in a way that clusters colors and intensities with similar properties
into rows and columns and also shows trends as you go from left to right across
groups and from top to bottom down periods.
After you have finished your periodic table,
have your teacher initial here ______.
- How did
you decide which colors to put in the same group?
- How did
you decide which colors to put in the same period?
- What
trend(s) do you have as you go down groups?
- What
trend(s) do you have as you go across periods?
Activity #9 – Color Coding
the Periodic Table
Color code the attached periodic table
according to the instructions below:
- Make the
dividing line (the zig-zag looking staircase)
between the metals and nonmetal more visible by using a highlighter or
colored pencil.
- Label each
side with arrows. “Metals” on the left and “Nonmetals” on the right.
- “Metalloids” are the elements that touch the
staircase line on one side. Draw diagonal lines in the metalloids boxes as
shown below and label “Metalloids”.


- Make a key at the bottom of the periodic table using four different
colors (see above).
- Color the boxes of the two liquid elements according to your
key. Their atomic numbers are 35
and 80.
- Color the eleven gas elements – atomic numbers 1, 2, 7, 8, 9, 10, 17, 18, 36,
54 and 86.
- Color the twenty synthetic or manmade
elements – atomic numbers 43, 61,
93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109 and 110.
- Use the fourth color to OUTLINE ONLY
the boxes containing the twenty-nine radioactive
elements. – atomic numbers 43, 61, 84, 85, 86, 86, 88, 89, 90, 91, 92, 93,
94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109
and 110.
- Near the top of the page, label the
groups and periods with arrows as shown below.

- Above group 1A, in the box, write the number of electrons in the outer shell
which is 1. Continue across the top with the remaining numbers of
electrons as shown in the table below.
|
column number |
1A |
2A |
3A |
4A |
5A |
6A |
7A |
8A |
|
# of electrons in the outer shell |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
- Write the number of energy shells in the boxes in the period
column. Hint: the top row is 1 and
the bottom is 7.
Activity #10 – Metals,
Nonmetals, & Metalloids
Read this website
and then write “metal”, “nonmetal”, or “metalloid” on the blanks next to the
properties.
- _______ semiconductors (are not the
best or worst conductors)
- _______ on
the right side of the periodic table
- _______ typically
shiny
- _______ good
conductors of heat and electricity
- _______ low
density
- _______ can
be shiny or dull
- _______ malleable
(bendable, can be pounded into sheets
- _______ will
corrode
- _______ dull
surface
- _______ high
density
- _______ poor
conductor of heat and electricity
- _______ are
along the staircase dividing line on the periodic table
- _______ most elements on the periodic
table are this
- _______ will
melt at low temperatures
- _______brittle
- _______ have
properties of both metals and nonmetals
- _______ only
melt at high temperatures
- _______
ductile (can be drawn into wires)
Activity #11 – Chemical
Families
Do the following crossword puzzles.
You will find the links to them on this page. After you are done, answer/do the
following:
1) In what group number, would you find the:
a) alkali metals _____
b) alkaline earth metals _____
c) chalcogens _____
d) halogens _____
e) noble gases _____
2) Label the above groups on your colored
periodic table.




Activity #12 – Review Games
and Worksheets
- Element Math Game - Choose the following options and
then click “I’m ready! Let’s start!:

When you finish, click “All Done” to get
your results. Show your results to your
teacher and have her initial here ___________.
- Element
Word Scramble - Play
the fifteen question version with clues.
After you finish, show your teacher the “Results” screen and have
her initial here: _________
- Periodic Table Jeopardy - After you finish, show your teacher
your score and have her initial here: _________
- ATOMIC
STRUCTURE, ISOTOPES and ELECTRON STRUCTURE - Enter your answers in the form
online as well as below so that you can check your answers.
|
15
2 2 2.7
2.8.5 21 21
22 26 26
2nd 3 30
4th 5 5th
7 8 8
9 atomic atomic
electrons electrons
electrons group iron
isotopes mass mass
neutrons neutrons nucleus
number period shell
shells three |
|
Q1(a) Atoms are made of fundamental
particles called protons (+), (0) and
(-). (b) The centre of the atom is
called the . (c) It consists of
protons and and contains most of the
mass of the atom.
|
- THE
PERIODIC TABLE - Enter your answers in the form online as well as below so that you
can check your answers.
|
+1
-1 0 1
1 1 2
2 7 alkali
atomic balloons before
boiling brittle
catalysts coloured
coloured
covalent densities
diatomic down dull
electronic electrons
floats gases gases
gases greater groups
halide halogens heat
heat heat high
higher hydrogen hydrogen
hydroxide inert ionic
left less less
less level liquid
liquid low low
lower mass mercury
metals metals metals
more more noble
non-metals non-metals
number period periods
poor properties right
same shiny similar
similarities single
solids transition unreactive |
|
Q1(a) The chemical elements
in the Periodic Table were originally arranged in order of the atomic . |