Prepared as part of an undertaking by the Allergy and Environmental Health
Association. Nova Scotia. Intervention Coalition on the proposed Sable Island
gas pipeline project
March 1997
Report prepared by: Karen McAllister, BSc(hon), M.E.S.
With assistence from: Helen Lofgren, M.A.
| Executive summary | |
|
PART I: Natural gas and people with allergies and chemical sensitivities: a special risk group | |
| 1.0 | Introduction |
| 2.0 | What is environmental illness and chemical sensitivity, and what causes it? |
| 2.1 | Environmentally induced illness/chemical sensitivity: a definition |
| 2.2 | Symptoms |
| 2.3 | Initiation of multiple chemical sensitivities: |
| 3.0 | Indoor Pollution and natural gas use |
| 4.0 | Natural Gas and people with chemical sensitivities: |
| 4.1 | Possibility of sensitization to methane and other hydrocarbons in natural gas |
| 4.2 | Odourants in the natural gas: their potential as a sensitising agent |
| 4.3 | Nitrogen Dioxide (NO2) |
| 4.4 | Aerosols, particulates and volatile organic compounds as products combustion in indoor natural gas appliances |
| 4.5 | Organometallic compounds |
| 4.6 | Benzene, toluene, ethylbenzene and xylene (BTEX) emissions |
| 5.0 | Cumulative effects of natural gas and its combustion products: |
|
PART II: Natural Gas: Evidence of toxicity in humans. A review of the current state of knowledge | |
| 6.0 | Natural gas: an overview |
| 7.0 | Hydrogen sulfide (H2S) |
| 7.1 | Human toxicity of hydrogen sulfide: |
| 7.2 | Effects of sour gas refineries on the health of neighbouring communities: |
| 8.0 | Naturally Occurring Radioactive Material (NORM) |
| 8.1 | Health effects of Radon |
| 8.2 | Radium and radon in petroleum and natural gas |
| 8.3 | Radiation from domestic use of natural gas |
| 9.0 | Organometallic compounds in some natural gas deposits |
| 9.1 | Human toxicological effects of organometallic compounds |
| 9.2 | Domestic Exposure |
| 10.0 | Odourants: |
| 11.0 | Products of combustion of natural gas |
| 11.1 | Carbon Monoxide (CO) |
| 11.2 | Nitrogen oxides (NOx) |
| 11.2.1 | Gas appliances and NO2 exposure |
| 11.2.2 | Health effects of indoor exposure to NO2 |
| 11.2.3 | Children's health and NO2 |
| 11.2.4 | Effects of indoor exposure to NO2 on the development and severity of allergy |
| 12.0 | Particulates and Volatile Organic Compounds (VOC) produced from natural gas combustion from appliances |
| 13.0 | Occupational Risk |
| 14.0 | Recommendations and Conclusions |
Figures and tables: | |
| Figure 1: | Observations concerning multiple chemical sensitivity |
| Figure 2: | Adverse effects of natural gas; letter from G.H. Ross, M.D. (1997) |
| Figure 3: | Daily fluctuations of indoor NO2 concentrations in a house with a gas stove |
| Table 1: | Concentration of metals in oxide coatings of gas burners in kitchens |
| Table 2: | Measurements of fine particulates released from various gas appliances |
| Table 3: | Fine particle emission rates for organic compounds released from natural gas home appliances |
From a health perspective, natural gas should not be used as a fuel in homes, public buildings, factories, businesses, shopping centres of other populated areas. Furthermore, gas pipelines should be avoided in any neighbourhood where people work, live or play. However, the use of natural gas for generation of electrical power to be transported to the home from generators not located in populated areas may be a cleaner fuel option than the existing coal or oil generators.
It is the position of the Allergy and Environmental Health Association - NS & NB that the proposed Sable natural gas pipeline project should not be approved until it has been demonstrated that the use of natural gas will not harm the health of sufferers of environmental illness and will not contribute to the development of new cases of environmentally illness and chemical sensitivity in the still healthy population. To date, no such evidence of proof has been tabled into this environmental assessment process.
This report outlines the potential health effects of using natural gas as a source of energy and fuel, primarily focusing on its contribution to indoor air pollution and subsequent health considerations. Special considerations are given to the potential health consequences of using indoor natural gas appliances for individuals with environmental and chemical sensitivities, asthma or allergies.
The first part of the report deals specifically with potential health effects of the use of natural gas for people with environmental illness, chemical sensitivities, asthma and allergies. It is estimated that 20 percent of the Canadian population is unusually sensitive to chemicals and allergens (CMHC 1994). Part 1 of this report examines the potential for natural gas and its products to contribute to the development of chemical sensitivity, asthma and allergy. It includes a description of environmental and chemical sensitivies, and their suspected causes and covers the potential health impacts of the individual components of natural gas and its combustion products on individuals with environmental sensitivities. There is very little information concerning this in the standard medical literature. Therefore, much of this section is based on informed clinical experience as well as direct observation of those with environmental illness. The only speculation is in the details. This emphasizes the need for further scientific and medical study of environmental illness, and on the effects of natural gas on environmentally sensitive individuals.
Part 2 of this document deals with the more traditionally considered health effects of natural gas, and reviews the current state of medical and scientific knowledge about the health effects of compounds present in natural gas and the products of combustion. This section provides background and support for the suspected health issues on the use of natural gas for individuals with environmental sensitivities and the development of chemical sensitivity, as presented in Part 1. Part 1: Natural gas and people with allergies and chemical sensitivities: a special high risk group
Although not much is conclusively known about the exact triggers for environmentally induced illness and chemical sensitivity, it is generally agreed that these illnesses have a toxic basis, and that low-level exposure to man-made and biological chemicals present in ambient levels in the environment can and are inducing symptoms in increasing numbers of individuals. Given the growing prevalence and serious and debilitating nature of environmental illness, lack of scientific certainty about its exact causes cannot and should not be a reason for dismissing this as an issue. A precautionary approach is essential when dealing with any developments or proposals for activities which may exacerbate illness in individuals who are already afflicted with chemical sensitivities, asthma and allergies, or which may induce new cases of environmental illness in the population.
Back to Contents
Figure 1 : Observations concerning multiple chemical sensitivity. From: (Henry et al 1991)
1. Symptoms involving virtually any system of the body or several simultaneously;
2. Differing symptoms and severity in different individuals, even those with the same exposure;
3. Induction (i.e., sensitization) by a wide range of environmental agents;
4. Subsequent triggering by lower levels of exposure than those involved in initial induction of the disease and ongoing lowering of threshhold;
5. Concomitant food intolerances, estimated to occur in a sizable percentage of those with chemical sensitivities;
6. "Spreading" of sensitivity to other, often chemically dissimilar, substances. Each substance may trigger a different constellation of symptoms;
7. Adaptation (masking), i.e., acclimatization to environmental incidents, both chemical and food, with continued exposure; loss of this tolerence with removal from the incident(s); and augmented response with re-exposure after an appropriate interval (e.g., 4-7 days);
8. An apparent threshhold effect referred to by some (including certain traditional allergists) as the patient's "total load".
Once sensitized, repeated triggering leads to progressive sensitization, and the individual responds adversely to an increasing number of substances in addition to the initiating chemical (Rossi 1996; Bell 1996). Direct olfactory stimulation is one likely route of exposure to sensitizing agents (Henry et al 1991). It is argued that the body's resistence mechanisms break down as repeated toxic environmental exposures occur (Rea 1992:10). Chemical exposure may adversely affect adaptive mechanisms, leading to illness (Henry 1991).
It is believed that several physiological and psychogenic mechanisms operate together in the development of chemical sensitivity disorder. The immune system, the limbic system (part of the brain) and enzyme detoxification mechanisms are thought to be involved (Henry 1991). It is not possible to predict who will develop environmental illness or chemical sensitivity. Everyone is vulnerable, although it is suspected that stress, genetics, hormones, nutrition, toxic exposure and living and working environments affect individual susceptability to the disorder.
Allergies to chemicals are a small component of chemical sensitivity, but the actual syndrome is much broader and more complex (Rea 1992:8). These allergies are a combination of immune response to chemical stimuli, as well as toxic responses not mediated by the immune system (Rea 1992:8).
Substances which are common irritants for sensitized individuals include tobacco smoke, perfumes, soaps, petrochemical substances (including natural gas), volatile organic compounds, cleaning agents, pesticides, and even coffee, alcohol, and foods which were previously tolerated (Rossi 1996). The effects are often debilitating, and victims severely affected are often unable to function in the public environment.
The use of natural gas as fuel for stoves, furnaces and other appliances contributes substantially to indoor air pollution. In Canada Mortgage and Housing Corporation's (CMHC) Clean Air Guide (1993), natural gas appliances (gas water heaters, furnaces, unvented space heaters and cook stoves) are identified as significant contributers of chemical contamination in the home. CMCH recommends replacement of these with electrical appliances (CMHC 1993:12).
Natural gas in the home contributes a number of different pollutants. These include products which exist in the gas itself (odourants, gas hydrocarbons, toxic organometallic complexes and radioactive radon), as well as products of incomplete combustion (nitrogen dioxide, carbon monoxide, fine organic particulates, polycyclic aromatic hydrocarbons (PAHs) and a small amount of volatile organic compounds (VOCs)). These pollutants have health implications as individual substances, as well as in combination and synergistically with each other and with other indoor pollutants. The general health implications of each of these individual substances is addressed in part 2 of this document. The health issues specifically pertaining to the development and exacerbation of environmental illness are addressed in the following section.
"In both Randolph's and our combined series of 47,000 patients, the most important sources of indoor pollution responsible for generating illness were the gas cook stoves, hot water heaters, and furnaces." (Rea 1994:706).
Dr. Gerald Ross of the Environmental Health Centre in Dallas writes that
| "...natural gas has been found to be one of the most important sources of indoor air pollution and sensitizing agents..." (refer to Figure 2, Ross 1997). |
| "We have seen thousands of patients who have complex allergies and sensitivities. Frequently, they will have only limited success with their treatment programs, if they are living in a home that has natural gas or if they are in an area where there is natural gas transportation or leakage" (Ross 1997). |
Removal of any indoor combustion products in the home, including and especially natural gas stoves and heaters, is one of the first recommendations for treating people who have multiple chemical sensitivities (Ross 1992; Rogers 1986:389-90).
Dr. T.G. Randolph, the founder of the clinical ecology movement, recognized the contribution of natural gas to health problems in chemically sensitive individuals, and to the initiation of chemical sensititivities as early as the 1950s (Randolph 1981). His work is a foundation for investigation into environmentally-induced illness. In his book, "An alternative approach to allergies", Randolph states,
| "Natural gas is advertised as the "clean fuel". This may be so from the point of view of visible smog-producing residues, but for the chemically susceptible individual this gas may be the worst form of fuel." (Randolph 1981:86) |
He further states that
| "Perhaps one of the most surprising aspects of this gas problem is the incredible sensitivity of some people to its presence. Merely shutting off a gas range is not enough to bring relief to such patients. The gas stove must be completely removed from the premises. This is because even a non-working range continues to give off odors from gas which it has absorbed over the years." (Randolph 1981:87) |
In his clinical studies, Randolph found that removal of gas ranges from homes resulted in an improvement of the health of all family members, not just those who were chemically sensitive (Randolph 1981:87).
Sales natural sales gas, as well as its impurities, additives and products of combustion, can have a potentially negative impact on both "healthy" and on immunologically compromised, chemically sensitive, asthmatic or allergic individuals. The individual substances in and produced by combustion of natural gas may have health consequences, and cumulatively, these substances can have additive or enhanced effects. Because of the difficulty in studying the cumulative effects of chemicals and other substances, most studies look at individual responses. However, often it is the cumulative low-level exposures or a series of brief repetitions of higher exposures to multiple substances which creates health problems. Furthermore, exposure to multiple compounds may have synergistic effects. Embryonic and childhood exposures may create developmental problems, and the effects of chemicals on the reproductive system may have transgenerational consequences.
Back to Contents
Figure 2: Adverse effects of natural gas; Letter from Dr. G.H. Ross, M.D. (1997)
January 3, 1997
RE: Adverse Effects of Natural Gas
To Whom It May Concern
Based on the work of pioneering allergists and specialists in Environmental Medicine, natural gas has been found to be one of the most important sources of indoor air pollution and sensitizing agents, that are commonly found in many human environments.
The most common indoor sources of this pollution are gas cook stoves, hot water heaters and furnaces. Traditionally, natural gas is a pollutant chemical that can worsen both classical allergy and chemical sensitivity. This effect has been seen mostly in areas where natural gas is in widespread use.
We have seen thousands of patients who have complex allergies and sensitivities. Frequently, they will have only limited success with their treatment programs, if they are living in a home that has natural gos or if they are in an area where thiere is natural gas transportation or leakage.
Sincerely,
Gerald H. Ross, M.D., C.C.F.P, D.I.B.E.M, D.A.B.E.M, F.A.A.E.M., President, Americal Academy of Environmental Medicine.
There is some evidence which suggests that hydrocarbons may act as hormone (endocrine) disruptors (Colborn et al 1996). The possibility that natural gas may also have a hormonal influence can not be discounted.
Clinical and epidemiological experience indicate that chemically sensitive people react adversely and severely to chemicals at concentrations which are expected to invoke only a mild to moderate odour sensation (Cone and Shusterman 1991). Individuals with asthma have identified odour as a trigger of asthmatic attacks (Cone and Shusterman 1991). It is estimated that the population prevalence of people sensitive to odours is greater than 15 percent (Cone and Shusterman 1991). Adverse reactions to odours at concentrations far below those which would cause irritation in a non-sensitized individual is a characteristic symptom of chemically sensitive individuals. Individuals with Multiple Chemical Sensitivity have symptomatic reactions to compounds at least two orders of magnitude lower than the established threshold for acute health effects (Cullen 1994:670).
It is known that mercaptan odours can cause nausea and headaches in healthy individuals (Sax and Lewis 1987:600). It is predictable and reported anecdotally that the response of chemically sensitive individuals to these odours is much greater.
Many of those afflicted with chemical sensitivities strongly attest that odours are the very worst triggers for causing a reaction. Some can suffer debilitating headaches, nausea, cognitive impairment, etc. from exposures as minute as a one story elevator ride or entering a room that a perfumed person has passed through, or picking up a book or telephone that a perfumed person has handled.
Dalhousie University, various hospitals, schools and many workplaces are instituting "No scents" policies in response to public and employee demand. The NS Nurses Union has spearheaded a highly successful "No scents is good scents" campaign. Some California communities have insitituted scent free municipal by-laws.
Another concern about mercaptans in natural gas is that the development of sensitivity to odours is suspected to be a contributing factor in the onset of chemical sensitivity (Cone and Shusterman 1991). Repeated exposure to a chemical can cause "acquired intolerance" or adaptation and loss of ability to smell the odourant. "Behavioural sensitization" is a recognised response in individuals who are subject to acute overexposure of an irritating chemical. After initial exposure, the individual reacts to levels which were previously tolerated (Cone and Shusterman 1991). These mechanisms overlap those described for the triggering of multiple chemical sensitivity disorder and "sick building" syndrome, raising questions about whether these illnesses are odour triggered in some cases (Cone and Shusterman 1991).
One of the key researchers in the sensitization effects of odour, Dr. Iris Bell, PhD, M.D., states that odours
| "can directly influence the function of a wide variety of central nervous system reactions. It might be speculated that specific odours could excessively stimulate or depress certain [brain] circuits to produce mannic depressive swings of mood seen in some ecological patients." (cited in Randolph 1981:226). |
Although mercaptans have not been evaluated for their sensitizing capacity, it is known that repeated exposure to ethyl mercaptan is subject to rapid adaptation in some people, who are no longer able to detect it by scent (Cone and Shusterman 1991). It seems logical that the reverse can also be true, and that mercaptans in natural gas have the potential to be sensitizing agents.
Mercaptans capture mercury. Because mercury is a sometimes a component of natural gas, it may become affixed to the mercaptans and breathed in with the odour, thereby potentially increasing one's burden of highly toxic mercury.
It is widely recognised that, aside from cigarette smoke, gas stoves and other gas-fired appliances are the primary contributors to indoor nitrogen dioxide NO2 concentrations (Jarvis et al 1996; Hansen 1991:61; Lambert and Samet 1996:790; Seinfeld 1986:58; Infante-Rivard 1993). Evidence suggests that NO2 generated from indoor gas appliances contributes a multitude of respiratory health ailments. These include:
1. respiratory tract inflammation and impairing lung function (Jarvis et al 1996; Lambert and Samet 1996:790);
2. increased risk of asthma-like symptoms, including wheezing, waking with shortness of breath, and asthma attacks (Jarvis et al 1996). This is especially true for women who cook with gas, and children (Jarvis et al 1996);
3. decreased resistence to lung bacterial infections because of decreased lung defense mechanisms (Seinfeld 1986:58; Hansen 1991:61; Jarvis et al. 1996;Infante-Rivard 1993; Lambert and Samet 1996:789);
4. adverse effects on animal immune systems (Seinfeld 1986:58; Lambert and Samet 1996:789);
5. exacerbation of asthma and lung disease, especially among children (Brauer et al 1996; Hansen 1991:61; Jarvis et al 1996);
6. acts as an adjuvant, and contributes to the development of allergy to other substances (Tunnicliffe et al 1994; Henry et al 1991).
The effects of such respiratory ailments will likely be enhanced in individuals who are chemically sensitive or environmentally ill.
There is some evidence that ambient levels of indoor NO2 is able to enhance or potentiate the onset of allergic reactions to other substances (Tunnicliffe et al 1994; Henry et al 1991). Furthermore, NO2 is thought to be able to increase sensitivity and allergic response to allergens (Tunnicliffe 1994).
The relationship between NO2 and the development and severity of allergy indicates that NO2 facilitates the sensitization process in the development of chemical and other environmental sensitivities. It appears likely that repeated exposures to NO2 and allergens have an additive effect (Tunnicliffe et al 1994).
Fine carbon compounds and polyaromatic hydrocarbons (PAHs) may also have a role in sensitization and allergy development. It is established that small, respirable particles are breathed deep into the lungs, and can bypass respiratory defense mechanisms, making them a greater hazard than larger particles (Hansen 1991:281). These have a negative effect on the immune system, irritate lung tissue, and aggravate existing repiratory disease (Hansen 1991:281). It is also thought that small organic compounds have a role in chemical sensitisation. The aggravating effects of these compounds will likely have an adverse effect on individuals with environmental sensitivities, and possibly contribute to the development of further sensitization.
During the E.I.A. hearings for the defeated Halifax garbage incinerator, it was established that micron-sized particles formed during combustion become adsorption sites for chemicals present in combustion air. The chemicals thus concentrate into micro-particulates where whey are increasigly likely to react with each other, especially when metals are present to catalize reactions. The smaller the particulate, the more surface area for this process per measured and reported mass. These small particulates are often invisible to the eye. All of the precursor elements and conditions for this are present in natural gas combustion.
A study in Poland found that toxic heavy metals are transported via natural gas and accumulate on gas burners (Kucha et al 1993). This raises concerns about possible adverse health effects from these toxic methylated metallic compounds. Studies have not yet been undertaken on the health effects of metalic components in natural gas.
In the event that the natural gas deposit off Sable Island contains similar compounds, the use of this natural gas in homes is a potential source of bio-accumulating toxic substances. People with chemical sensitivities have measurable levels of toxins in their blood and tissues (Ross 1992). It is suspected that certain individuals have an impaired ability to detoxify chemicals which accumulate in their tissues (Ross 1992). These organometals could increase their toxic load. In addition, the potential sensitizing effects of these compounds on individuals who are not yet sensitised are unknown.
Radon, nitrogen dioxide, and fine particulates emitted from natural gas combustion all have health consequences for lung function and respiration. In combination, these effects may be additive or compounding. NO2 is suspected to potentiate allergic development and response to other compounds. It may also increase sensitivity to other components of natural gas and its combustion products. There is potential for methylated heavy metals in natural gas to bioaccumulate, creating potential for neurological damage. Odourants such as mercaptans are suspected of playing a role in the initiation of chemical sensitivities.
The individual toxic actions of these various components of natural gas are all potentially significant for human health. When acting in combination, these toxic effects may be additive or compounding.
The use of natural gas in the home, as fuel for heating or cooking, is a health issue for healthy individuals and especially for people with environmental illnesses, allergies, and chemical sensitivities. The concentration of combustion products from natural gas appliances in indoor environments has wide-ranging health consequences. Furthermore, the natural gas itself causes adverse reactions in sensitive individuals.
| "Combustion gases and unburned fuel (including the additives) from fossil fuel appliances can be a major source of contaminants in the home. Sufficient evidence has accumulated on the negative impact on health of using open flame gas stoves". (Canadian Mortgage and Housing Corporation 1993) |
Characteristics of natural gas which are of concern to human and animal health include: 1. its highly flammable and explosive nature,
2. its asphyxiant properties,
3. products generated from the combustion of natural gas, especially as contributors to indoor air pollution. (NO2, nitric acid when NO2 combines with moisture, carbon monoxide, polycyclic aromatic hydrocarbons (PAH), volatile organic compounds, fine organic particles),
4. the presence of radioactive elements (radium and radon) in natural gas,
5. the presence of highly toxic H2S in certain gas deposits, and the environmental and health effects of refining such sour gas,
6. the presence of trace amounts of toxic metals in some gas deposits,
7. the toxicity of odourants added to the sales gas, especially with respect to people with multiple chemical sensitivities, and
8. the sensitizing effects of natural gas.
Some natural gas deposits have a high concentration of hydrogen sulfide (H2S), which poses significant environmental and health concerns. Hydrogen sulfide is a colourless, highly toxic gas with a strong smell of rotten eggs (Gosselin et al 1984:198). It is produced naturally by decaying organic matter, and is also released from sewage sludge, oil refineries, liquid manure, sulphur hot springs, and natural gas.
Although H2S has a strong odour, continual low level exposure leads to loss of the sense of smell. This makes it possible for humans to be unknowingly exposed to dangerous levels. Low level of H2S result in irritation of the eyes, nose and throat. Moderate levels can cause headache, dizziness, vomiting, as well as cough and difficulty in breathing. High levels can cause shock, convulsions, coma and death. Survivors of acute toxic exposure to H2S sometimes experience neurological dysfunction, such as amnesia, tremor, disturbance of equilibrium, or more serious brain damage, as well as persistent ill-health following exposure (Gosselin 1984:200). Although acute toxicity for higher concentrations of H2S is established, there are few studies on chronic low level exposure to H2S (Schechter 1989). This is a serious deficiency.
Accidental release of concentrated H2S may result from infrequent blowouts of natural gas wells which have high levels of H2S. Although the risk is low, the acute toxicity of H2S implies that this may have a serious affect on the health of nearby human populations (Layton and Cederwell 1986).
It has been shown that SO2 exposure can lead to lung cancer in humans (Schechter 1990). A study on respiratory problems in the same region showed an increased prevalence of respiratory symptoms in children with greater environmental exposure to gas plant emissions (Dales 1989).
Some Albertans and others are seriously challenging these assumptions and are claiming that they and their livestock, plants and property are being seriously harmed by gas pollution. This has attracted considerable attention, including feature articles in the Globe and Mail.
Naturally Occurring Radioactive Material (NORM) is found in soil, water, petroleum, natural gas, coal, lignite, phosphate, geothermal waste, wastewater, humans and animals (Wilson 1994:1). One of the most common NORMs is radon (Rn222), a colourless, odourless gas produced from Radium (Ra226) decay and a decay product of radioactive uranium (Wilson 1994:84). Ra226 has half life of 1,602 years (Wilson 1994:43), while radon has a short half-life of only 3.8 days, after which it emits 4 highly reactive "radon daughters" (Neely 1994:136).
Radon is commonly found in natural gas deposits, ground water and soil, and is a common indoor air contaminant in basements where it seeps through the foundation and is concentrated in enclosed spaces. Nova Scotians are already exposed to radon in soil and water. Because of the geology of Nova Scotia, it is highly likely that natural gas deposits off Sable Island also contain high levels of radium and radon.
Some deposits of natural gas have been found to naturally contain high concentrations of heavy metals, including lead (Pb), Copper (Cu), Mercury (Hg), Silver (Ag), and Arsenic (As) (Kucha et al 1993; Mohan et al 1992; Boogaard and Journ‚e 1996). It is suspected that these metals are present in the form of organometallic compounds, such as (CH3)3As, (Ch3)4Pb and (Ch3)2Hg. One study on natural gas produced in Abo, New Mexico, found As concentrations of 0.2 to 2 micrograms/litre gas in the form of low molecular weight organoarsines, with 55-80% of these being (CH3)3As (Mohan et al. 1992). White solids deposited near pressure regulators of the pipeline were found to be organoarsenic sulfides, (CH3)3AsS (approxiomately 55%), and (CH3)2As(C2H5)S (approximately 45%) (Mohan et al 1992).
Dimethylmercury ((CH3)2Hg) is a particularly dangerous organometallic compound because of its volatility and high lipid solubility, resulting in a high level of absorption and tendency to bioaccumulate and biomagnify in the fatty tissues of mammals and fish (Manahan 1991:133). Methyl mercury can be absorbed through inhalation because of its high vapour pressure, and can also be absorbed through the skin. The uptake of Methyl mercury is nearly 100% in the gastrointestinal tract (Manahan 1991:133). Mercury has neurotoxic and reproductive effects on humans. There is no human requirement for mercury, nor any safe level.
Organoarsenic compounds are most toxic when they are broken down metabolically, resulting in highly toxic inorganic forms of As.
Back to Contents
Table 1: Concentration of metals in oxide coatings of gas burners in kitchens (Kucha et al 1993)
| Locality (town) | Pb(p.p.m.) | Ag(p.p.b) | Cu(p.p.m.) | As(p.p.m.) | Distance to Deposit (km) |
| Siekierki | 112 | 98 | 34 | 15 | 20 |
| Buk | 491 | 1,082 | 57 | 32 | 5 |
| Buk | 511 | 1,321 | 61 | 45 | 5 |
| Poznan | 134 | 82 | 21 | Less than 5 | Approx. 100 |
| Vienna | 1,283 | 1,136 | 61 | ? | 3,000 |
In this case study, the natural gas deposit providing the fuel was known to contain high levels of Pb, Hg, Cu, Ag and As (Kucha et al 1993). The collection and subsequent analysis of oxide coatings on domestically-used gas burners found high levels of Pb, Hg, Cu and Ag, as well as some As, with concentrations corresponding to the distance from the gas source (refer to Table 1). It is suggested that these metals were transported to the homes in the form of volatile organometallic complexes such as (CH3)4Pb. The high toxicity of (CH3)4Pb and methyl mercury (CH3)2Hg poses a significant health concern, since methylated compounds of these metals are much more toxic than the metals alone. The study postulates that mercury content of kitchen air during cooking may exceed safety regulations by 23-26 times. Similar studies have not yet been done on lead. Further analysis of metal precipitates found on burners and in natural gas is needed.
Analysis and studies on the possible health effects of the transport of oragnometallic complexes into homes should be done before the Sable Gas Project's environmental impact assessment is approved. The results need to be part of the material before the assessment is scrutinized.
Because natural gas itself has no odour, small amounts of odourants are added to the sales gas so that gas leaks can be recognised before concentrations reach a dangerously flammable or explosive level (intended concentration is such that odour will be detected at approximately one-fifth of the lower flammability limit, or about 4-24 grams of odourant per km3 gas (Environment Canada 1984:30). These odourants are sulfur compounds, and include mercaptans (ethyl mercaptan (methanethiol), methyl mercaptan, isopropyl mercaptan, T-butyl mercaptan), thioesthers and thioaromatics (Environment Canada 1984:30). The potential toxicity of these odourants raises issues concerning the safety of the use of natural gas in homes, especially for people with multiple chemical sensitivities. Adequate testing on odourant toxicity has not been conducted using people with chemical sensitivity. This should be part of the impacts assessment of the proposed Sable pipeline project. AEHA-NS requests for funding for such testing were denied.
Mercaptans are a common air contaminant. They contain sulfur and are able to capture elemental mercury. Exposure to Mercaptan odours can cause nausea or headaches. In high concentrations, mercaptans can cause cold extremities and rapid pulse, and may induce unconsciousness with cyanosis (Sax and Lewis 1987:600), or even death (Gosselin et al 1984:116). Mercaptans are dangerous when heated to decomposition because they emit highly toxic SOx fumes. Furthermore, they will react with water, steam or acids to produce toxic and flammable vapours, and can react violently with oxidizing compounds (Sax and Lewis 1987:600).
Methanethiol (also known as methylmercaptan, mercaptomethane, thiomethyl alcohol) is a mercaptan gas which is commonly used as an odourant in natural gas. Its unpleasant odour is detectable by most people at 1 part in 140 million (Gosselin et al 1984:116), however it may be detected at much smaller concentrations by highly sensitive individuals. Animal toxicological studies have shown that 0.16% methanethiol, 3.3% ethanethiol or 9.6% dimethyl-sulfide induce coma in 50% of rats exposed during a 15 minute period. However, the effects wear off after 30 minutes away from the gas. Human toxicity is proven through the case of a man found comatose within an hour after respiratory exposure to an unknown concentration of methane-thiol. The man experienced severe transient haemolytic anemia, and died 28 days after exposure, never recovering from the coma. It is suggested that methanethiol toxicity is similar to that of hydrogen sulfide (Gosselin et al 1984:116).
Another mercaptan used as an odourant is mercaptoethanol C2H6OS (also known as 2-thioethanol, ethylmercaptan). This is a severe eye and skin irritant, and is poisonous on ingestion, through skin contact, and by intravenous and intraperitoneal routes. It is highly flammable and is dangerous when heated to decomposition because it emits SOx fumes (Sax and Lewis 1987:601). Over time, many people become less sensitive to the smell of ethyl mercaptan, reducing its warning potential over time when it is used as a gas odorizer (Cone and Shusterman 1991). Decreased ability to recognise certain smells is often characteristic of the development of increased chemical sensitivity in an individual.
Carbon monoxide (CO) is an odourless, tasteless and colourless gas (Hansen 1991: 60-1,259; Seinfeld 1986:54-56, Lambert and Samet 1996:788-9). It is an asphyxiant, and binds to haemoglobin with about 200 times the affinity of oxygen, thereby reducing oxygen transport to the tissues (Hansen 1991:60,259; Lambert and Samet 1996:788). Acute exposure to CO can be fatal (Seinfeld 1986:55). Early symptoms of CO toxicity include dizziness, dull headache, nausea, ringing in the ears, and a pounding heart (Hansen 1991:60,259). Young adults with low blood carboxyhaemoglobin levels of only 5% have shown neural and behavioural effects, such as reduced hand-eye coordination and reduced visual sensitivity (Seinfeld 1986:55). Continued exposure may lead to unconsciousness and damage to the central nervous system, brain and circulatory system. Individuals with asthma, anaemia, heart disease or hyper-metabolic diseases, as well as young children, are most susceptible to CO poisoning (Hansen 1991:61,259).
Carbon monoxide poisoning is a frequent occurrence in industrialised countries (Risser and Schneider 1995). In the United States, it is estimated that accidental CO poisoning is responsible for about 900 deaths per year (Lambert and Samet 1996:788). Most of these are automobile exposures, however a proportion are from indoor CO sources. It has been suggested that continuous low level exposure to CO in indoor environments can cause early symptoms of CO poisoning, such as headaches and dizziness. Most CO poisonings occur in the winter when indoor heating equipment are operating. Between 3-5% of the cases of headache and dizziness of patients in hospital emergency rooms during winter periods are thought to be attributed to CO poisoning in the home (Lambert and Samet 1996:788), and a forensic study of deaths by CO poisoning found a greater occurrence in the winter (Risser and Schneider 1995). There is evidence that the use of gas furnaces and stoves in residences contributes to chronic low level CO poisoning as well as fatal CO exposure (Lambert and Samet 1996:788; Risser and Schneider 1996).
There has been continual concern about the role of gas furnaces and stoves in incidents of CO poisoning in the home (Anonymous 1970; Anderson 1970). One study which considered accidental CO-related deaths in Vienna between 1984-1993 suggests that improperly maintained and vented gas heating appliances were the cause. Most deaths were from gas-fuelled water heaters, some of which were unvented or defective. Gas stoves also contributed to fatal CO poisoning. Most victims were 60 years or over and were over-using flueless gas water heaters during the winter period when rooms may not be aired sufficiently (Risser and Schneider 1995). It is suggested that in a well insulated apartment, even a well-maintained gas water heater can create CO problems (Risser and Schneider 1995).
Nitrogen oxides (NOx) are products of fossil fuel combustion, produced by automobiles, gasoline engines and by gas-fuelled appliances (Lambert and Samet 1996:789). These are irritant gases, and include the compounds NO, NO2, N2O, OONO, ON(O)O, N2O4, and N2O5 (Hansen 1991:60). From the perspective of human health, nitrogen dioxide (NO2) is the most important of these. NO2 is an oxidant gas which is soluble in tissues (Lambert and Samet 1996:789). It is known to irritate eyes, nose, throat, and lungs (Seinfeld 1986:58; Hansen 1991:61), and is believed to exacerbate asthma and allergy. At high concentrations causes extensive lung damage in animals and humans (Lambert and Samet 1996). A more detailed description of the health effects of NO2 is given later in this section.
One of the most important sources of NO2 in the home environment is gas-fired stoves (Jarvis et al 1996; Clausing et al 1986; Lambert and Samet 1996). Cooking with gas produces NO2, nitric oxide, nitrous acid and humidity, among other things (Jarvis et al 1996). In houses with gas stoves, average indoor air concentrations of NO2 during the winter months are estimated to be 1.5 to 2 times the outdoor concentration (Lambert and Samet 1996:789). NO2 is most concentrated in the kitchen, however gas stoves increase NO2 concentrations throughout the house (Jarvis et al. 1996). In a New Mexico study, indoor NO2 levels in homes with gas stoves were measured to be on average 34ppb in the kitchen and 24ppb in the bedroom (Lambert and Samet 1996:789). Ambient outdoor levels were estimated on average to be 15ppb, while indoor levels in homes with electric stoves measured significantly lower, with an average of 7ppb.
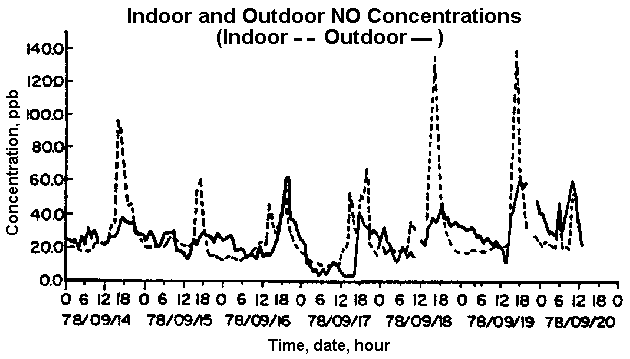
The concentration of indoor NO2 generated from gas stoves characteristically peaks during cooking times (refer to figure 3), and may reach transient levels as high as 1000ppb. These levels may be maintained for 20 to 60 minutes after cooking has stopped (Lambert and Samet 1996:789). Another study estimates transient cooking levels of NO2 to reach 400ppb (Tunnicliffe et al. 1994). Adults who spend time cooking with gas are exposed to short bursts of NO2 in greater concentrations than those experienced by other family members, and therefore are more likely to experience negative health effects from this exposure (Jarvis et al 1996; Brauer et al 1996). This has been substantiated by a study on the impacts of NO2 on women who cook regularly with gas stoves (Jarvis et al 1996).
Back to Contents
Figure 3: Daily fluctuations of indoor NO2 concentrations in a house with a gas stove (Walsh
et al 1984:65).

1. reduced defense against infection
2. exacerbation of asthma and other lung diseases, and
3. respiratory tract inflammation with manifestations of respiratory symptoms and lung function. (Lambert and Samet 1991:790).
The results of epidemiological studies concerning the effects of indoor NO2 on health are inconsistent and often conflicting. This could be owing in part to differences in research methodologies. Studies which monitor exposure to NO2 by measuring average indoor levels don't take into account differences in personal NO2 exposure of different household members nor the intermittent higher level exposure associated with gas cooking. Studies which mimic such chronic short exposures generally confirm that NO2 has an adverse affect on respiratory health (Jarvis et al 1996). Furthermore. in spite of conflicting research results, the many epidemiological studies which suggest that NO2 exposure is positively correlated with respiratory disease, especially among children, provide cause for concern.
Controlled human exposure studies and epidemiological studies in homes with gas stoves have indicated altered lung function and acute respiratory symptoms and illness (Hansen 1991:61). Several studies have shown a positive correlation between impaired lung function and respiratory illness in women who use gas stoves (Jarvis et al 1996). In a study by Jarvis et al (1996), it was shown that young women who used gas stoves and did most of the household cooking had reduced lung function and an increased risk of asthma-like symptoms, including wheezing, waking with shortness of breath, and asthma attacks. Women using gas stoves with extractor fans which removed the cooking fumes outside were equally likely to show symptoms as those whose stoves were not ventilated. More women than men experienced respiratory difficulties - an observation owing either to the possibility that women are more sensitive to NO2 emissions, or, as is more likely the case, that women have greater exposure to gas stove emissions because they are more often responsible for household cooking. Similar results were found in a study in Singapore, which showed that non-smoking women using gas stoves have more respiratory and lung problems than those who use gas infrequently or not at all (Jarvis et al. 1996 citing Ng et al 1993).
There is evidence to suggest that NO2 reduces efficacy of lung defences and increases susceptibility to pulmonary bacterial infection (Seinfeld 1986:58; Hansen 1991:61; Jarvis et al. 1996; Infante-Rivard 1993; Lambert and Samet 1996:789). Other studies have indicated effects on viral infection, lung defence mechanisms, and animal immune systems (Seinfeld 1986:58; Lambert and Samet 1996:789). The toxicological effects of NO2 raises concern about the possible variety of adverse health effects on humans, particularly its potential for decreasing resistance to pulmonary disease, exacerbating asthma and allergic reactions, and causing respiratory tract inflammation impairing lung function (Lambert and Samet 1996:790). NO2 is thought to exacerbate asthmatic response. Lambert and Samet state that "persons with asthma have a chronic inflammatory process associated with increased responsiveness of the airways to environmental stimuli, and NO2 could plausibly worsen this process" (Lambert and Samet 1996:790).
Combustion sources - gas-fired appliances, tobacco smoking, kerosene heaters - all produce varying amounts of polycyclic aromatic hydrocarbons (PAHs) and carbonaceous particles from incomplete combustion (Sexton 1986; Koutrakis et al 1991). Combustion of natural gas in home appliances creates emissions of fine particulate and gaseous matter (Rogge et al 1993). It has been demonstrated that a small number of fine particles and low levels of solid particulates are emitted from natural gas combustion, as well as semi-volatile compounds that partition between the gas phase and particle phase (Rogge 1993; Sexton 1986). Organic carbon compounds constitute the majority of fine particles emitted from natural combustion (Rogge 1993).
One study characterized trace metals, ionic species, and organic and elemental carbon compounds emitted from a vented natural gas-fired space heater and a natural gas fired water heater (refer to Tables 2 and 3) (Rogge et al 1993). Combustion exhaust was shown to include 16 - >27 carbon compounds, including alkenes, alkanes and alkanoic acids. These are suspected to have a biogenic origin and to be introduced into furnaces or appliances from ambient air. The experiment determined that the dominant organic material (22.5 % of the aerosol mass) produced from home natural gas appliances are polycyclic aromatic hydrocarbons (PAHs) and oxygenated polycyclic aromatic hydrocarbons (oxy PAHs), including polycyclic aromatic ketones (PAK) and polycyclic aromatic quinones (PAQ). In addition, thia- and aza- arenes are produced by the incorporation of nitrogen and sulfur from combustion into the PAHs generated (Rogge et al 1993). It was concluded that these substances were derived almost exclusively from natural gas combustion, and were not introduced from ambient air. The exact particles found are listed in Table 2. A similar study which measured particulate emissions from a natural gas stove found no PAHs emitted, however found sulfur and silicon (Sexton 1986).
The presence of nitrogen containing aza-arenes, and sulfur containing thia-arenes in exhaust emissions from natural gas fired space and water heaters is of particular health concern because they are suspected of being carcinogenic, and have tested positive in the Ames mutagenicity and in animal carcinogenicity tests (Rogge 1993).
Back to Contents
Table 2: Measurements of fine particulates released from various gas appliances (Rogge et al 1993)
| Compound | Emission Rates (pg/kJ) | Compound ID | ||
| HEPA- Filtered dillution air | Space and Water Heater | |||
| n-Alkanes | First Filter | Backup Filter | ||
| Nonadecane | 3.2 | N/A | 4.6 | Positive |
| Eicosane | 8.4 | 13.3 | 12.0 | Positive |
| Heneicosane | 16.8 | 50.9 | 28.6 | Positive |
| Docosane | 13.1 | 108.9 | 39.8 | Positive |
| Tricosane | 7.1 | 164.6 | 28.8 | Positive |
| Tetracosane | 3.2 | 112.4 | 6.2 | Positive |
| Pentacosane | 1.4 | 55.2 | 2.9 | Positive |
| Hexacosane | 1.2 | 23.8 | 1.5 | Positive |
| Heptacosane | 0.75 | 32.2 | N/A | Positive |
| Octacosane | 0.84 | 12.7 | N/A | Positive |
| Nonacosane | 0.61 | 50.2 | N/A | Positive |
| Triacontane | 0.24 | 6.6 | N/A | Positive |
| Hentriacontane | 0.26 | 16.6 | N/A | Positive |
| Dotriacontane | N/A | 1.1 | N/A | Positive |
| Tritriacontane | N/A | 0.70 | N/A | Positive |
| Total Class Emission Rate | 57.1 | 648.2 | 124.4 | |
| n-Alkanoic Acids | ||||
| Octanoic Acid | 16.2 | 152.0 | 116.9 | Positive |
| Nonanoic Acid | 32.6 | 225.2 | 482.6 | Positive |
| Decanoic Acid | 2.9 | 119.5 | 131.3 | Positive |
| Undecanoic Acid | N/A | 43.2 | 19.4 | Positive |
| Dodecanoic Acid (Lauric Acid) | 6.9 | 120.7 | 56.2 | Positive |
| Tridecanoic Acid | N/A | 16.0 | 4.8 | Positive |
| Tetradecanoic Acid (Myristic Acid) | 3.7 | 59.6 | 29.9 | Positive |
| Pentadecanoic Acid | 0.96 | 14.3 | 8.5 | Positive |
| Hexadecanoc Acid (Palmitic Acid) | 9.7 | 310.4 | 56.8 | Positive |
| Heptadecanoic Acid | N/A | 12.5 | 4.1 | Positive |
| Octadecanoic Acid (Stearic Acid) | 2.6 | 81.8 | N/A | Positive |
| Nonadecanoic Acid | N/A | N/A | N/A | Positive |
| Eicosanoic Acid | N/A | N/A | N/A | Positive |
| Heneicosanoic Acid | N/A | N/A | N/A | Positive |
| Docosanoic Acid | N/A | N/A | N/A | Positive |
| Tricosanoic Acid | N/A | N/A | N/A | Positive |
| Tetracosanoic Acid | N/A | N/A | N/A | Positive |
| Pentacosanoic Acid | N/A | N/A | N/A | Positive |
| Hexacosanoic Acid | N/A | 5.3 | N/A | Positive |
| Heptacosanoic Acid | N/A | N/A | N/A | Positive |
| Octacosanoic Acid | N/A | 4.0 | N/A | Positive |
| Nonacosanoic Acid | N/A | N/A | N/A | Positive |
| Triacontanoic Acid | N/A | N/A | 1.4 | Positive |
| Total Class Emission Rate | 75.56 | 1165.3 | 910.5 | |
| Polycyclic Aromatic Hydrocarbons | ||||
| Phenanthrene | 0.20 | 6.3 | 3.2 | Positive |
| Anthracene | 0.22 | 1.9 | 0.26 | Positive |
| Fluoranthene | 0.19 | 685.3 | 251.7 | Positive |
| Pyrene | 0.58 | 759.3 | 146.0 | Positive |
| Benzacenaphtylene | N/A | 1.6 | N/A | Probable |
| 2-Phenylnaphthalene | N/A | 13.6 | 6.1 | Probable |
| Methyl(fluoranthenes, pyrenes) | N/A | 29.3 | 10.5 | Probable |
| Benzo(a)fluorene / Benso[b}fluorene | N/A | 13.3 | 1.1 | Positive |
| Benzo[ghi]fluoranthene | N/A | 397.7 | 130.7 | Probable |
| Benzo[c]phenanthrene | N/A | 125.5 | 48.9 | Positive |
| Benz[a]anthracene | N/A | 497.7 | 6.7 | Positive |
| Chrysene / Triphenylene | N/A | 1922.7 | 524.8 | Positive |
| Methyl(benz[a]anthracenes, chrysenes, triphenylenes) | N/A | 75.8 | 5.6 | Probable |
| Dimethyl(fluoranthrenes, pyrenes) | N/A | 139.7 | 9.0 | Probable |
| 1,2'-; 2,1'-; 2,2'- Binaphalenes | N/A | 139.7 | 9.0 | Probable |
| Benzo[k]fluoranthene | N/A | 393.8 | 6.0 | Positive |
| Benzo[b]fluoranthene | N/A | 284.4 | 5.4 | Positive |
| Benzo[e]pyrene | N/A | 116.0 | 1.9 | Positive |
| Total Class Emissions Rate | 1.19 | 5494.5 | 1157.86 | |
| Ploycyclic Aromatic Ketones and Quinones | ||||
| 9H-fluoren-0-one (Fluorenone) | N/A | 551. | 49.2 | Positive |
| 1H-phenalen-1-one | N/A | 618.4 | 3.0 | Positive |
| Methylfluorenones | N/A | 159.5 | 93.9 | Probable |
| 9,10-Phenanthrenedione (Phenanthrenequinone) | N/A | 12.1 | 4.5 | Positive |
| 9,10-Anthracenedione (Anthraquinone) | N/A | 1976.8 | 39.1 | Positive |
| 2-Methyl-9,10-anthracenedione (2-Methylanthraquinone) | N/A | 69.7 | 0.47 | Positive |
| Phenanthrone / Anthrone | N/A | 21.4 | 1.2 | Positive |
| 9H-xanthen-9-one (Xanthine) | N/A | 49.3 | 15.6 | Positive |
| 4-Cyclopenta[def]-phenanthren-4-one | N/A | 920.2 | 501.9 | Probable |
| 1H-benz[de]anthracen-1-one | N/A | 132.0 | 2.9 | Probable |
| 7H-benz[de]anthracen-7-one | N/A | 94.7 | 0.82 | Positive |
| Benz[a]anthracene-7,12-dione | N/A | 123.7 | 104.1 | Positive |
| Total Class Emission Rate | N/A | 4232.9 | 816.69 | |
| Aza and Thia Arenes | ||||
| Benzo[h]quinoline | N/A | 36.6 | 30.6 | Positive |
| Benzo[f]quinoline | N/A | 25.9 | 1.7 | Probable |
| Phenanthridine | N/A | 48.4 | 1.0 | Positive |
| Acridine | N/A | 7.4 | N/A | Positive |
| Aza arenes with MW203 (Isomers such as Indeno(1,2,3-i,i)isoquinolene, Acenapho(1,2-b)pyridine, and 4-Azapyrene) | N/A | 124.3 | 26.6 | Probable |
| Benzo[b}naphthothiophenes | N/A | 325.4 | 103.5 | Probable |
| Total Class Emission Rate | N/A | 568.0 | 163.4 | |
Back to Contents
Table 3: Fine particle emission rates for organic compounds released from natural gas
home appliances (Rogge et al 1993)
| APPLIANCE | PARTICLE EMISSION |
| Unvented and vented space heaters | 6-300 ng/kJ |
| ovens | <50 ng/kJ |
| stove top burners | 240-620 ng/kJ |
Certain Volatile Organic Compounds (VOC) are also produced in incomplete natural gas combustion (Lambert and Samet 1996:793). Some VOCs are carcinogenic and/or neurotoxic. It is thought that VOCs, through mucosal irritation and neurotoxic effects, play a role in "sick building" syndrome (Lambert and Samet 1996:793). Formaldehyde is among the VOCs produced from indoor natural gas production (Hansen 1991:269). Formaldehyde is highly reactive, and accute exposure causes eye, ear, throat, and nose irritation, as well as coughing, wheezing and skin rash (Hansen 1991:269). Formaldehyde is also known to induce severe allergic reactions.
Natural gas workers may be exposed to radon radiation in natural gas during processing, storing equipment, and cleaning of equipment (Gesell 1975; Nigro and Bunn 1996). They are also exposed to any heavy metals in the natural gas. A Dutch study considers the occupational health problems of natural gas workers exposed to mercury in gas deposits (Boogaard and Journ‚e 1996). Workers are potentially exposed to large concentrations of elemental Hg, especially when opening installations for inspection or maintenance operations. Air measurements established that exposure during maintenance operations was high, in spite of personal protection equipment, and that Hg concentrations in some areas exceeded the Dutch exposure limit value of 50 micrograms/m3. Other studies of individuals exposed to similar concentrations showed long term neural and tremor effects, and renal problems. There is a need to look also at the chronic effects of long-term, low-dose exposure. It has been shown that occupational exposure to chronic low levels or occasional igh levels of chemicals can induce chemical sensitivity.
A review of the potential health effects from the use of natural gas suggests that this should not be used as a source of indoor fuel; especially not in homes, offices, in public buildings such as schools, libraries, or hospitals, or in any building or neighbourhood where people live, work or play . This recommendation is backed by the report of Canada Mortgage and Housing Corporation (CMHC 1994) which recommends the replacement of indoor gas appliances and combustion sources with electrical appliances in order to reduce indoor air pollution. The potential severity of indoor pollution from natural gas on health, particularly its potential in contributing to the development of and exacerbating chemical sensitivity, environmentally-induced illness, asthma and allergy suggests that a precautionary approach is warranted.
It is the position of the Allergy and Environmental Health Association - NS and its coalition partners that the proposed Sable natural gas pipeline project should not be approved until it has been demonstrated that the use of natural gas will not harm the health of those afflicted with environmentally induced illness and will not contribute to the development of new cases of environmentally induced illness, chemical sensitivity, asthma and allergy in the still healthy population. Since no such evidence has been tabled in this environmental assessment process, AEHA-NS and its coalition partners do not recommend that the Panel grant approval to proceed with this project.
Although it is suggested that natural gas not be used as a fuel source within buildings, its use for producing electricity in generators away from homes and populated areas, to be transported into homes via the electrical power grid would be less potentially damaging from the perspective of inducing environmental sensitivities. The use of natural gas for generating electricity outside of the home is likely to be a healthier and cleaner source of power than the existing coal-fired or oil-fired generators.
Anonymous (1970) Natural gas: friend or foe? The Lancet 2(675):699-700
Bell, I.R. (1996) Clinically relevant EEG studies and pschophysiological findings: possible neural mechanisms for multiple chemical sensitivity. Toxicology 111:101-117
Boogaard, P.J. and Journ‚e, H.L. (1996) Effects of exposure to elemental mercury on the nervous system and the kidneys of workers producting natural gas. Archives of Environmental Health. 51(2):108-115
Brauer, M; Kennedy, S.M. (1996) Gas stoves and respiratory health. The Lancet 347:412
Canada Mortgage and Housing Corporation (CMHC) (1993) The clean air guide: how to identify and correct indoor air problems in your home. Ottawa: Canada Mortgage and Housing Association
Clausing, P.; Mak, J.K.; Spengler, J.; et al. (1986) Personal NO2 exposures of high school students. Environment International. 12:413-417
Colborn, T.; Dummanoski, D. and Myers, J.P. (1996) Our stolen future: are we threatening our fertility, intelligence, and survival? A scientific detective story. Dutton: Penguin Group
Cone, J.E.; Shusterman, D. (1991) Health effects of indoor odorants. Environmental Health Perspectives 95:53-91
Cullen, M.R. (1994) Low-level environmental exposures. Chapter 25 in: Rosenstock, L. and Cullen, M.R. Textbook of clinical and environmental medicine. Philadelphia, Pennsylvania: W.B. Saunders Company. pp. 667-672
Dales, R.E.; Spitzer, W.O.; Suissa, S. (1989) Respiratory health of a population living downwind from natural gas refineries. Am Rev Resp Dis. 139:595-600
Environment Canada. Environmental Protection Service (1984) Natural Gas: Enviro Technical Information for Problem Spills. Ottawa: Environmental Protection Service, Environment Canada
Gesell, T.F. (1975) Occupational radiation exposure due to 222Rn in natural gas and natural gas products. Health Physics 1995;29:681-687
Gosselin, R.E.; Smith, R.P.; Hodge, H.C. (1984) Chemical toxicology of commercial products. 5th edition. Baltimore: Williams and Wilkins
Hansen, S.J. (1991) Managing indoor air quality. Liburn, GA: The Faimount Press, Inc.
Henry, C.J.; Fishbein, L.; Meggs, W.J. et al. (1991) Approaches for assessing the health risks from complex mixtures in indoor air: a panel overview. Environmental Health Perspectives. 95:135-143
Infante-Rivard, C. (1993) Childhood asthma and indoor environmental risk factors. American Journal of Epidemiology. 137(8):834-44
Jarvis, D; Chinn, S.; Luczynska, C. et al. (1996) Association of respiratory symptoms and lung function in young adulats with use of domestic gas appliances. The Lancet. 347:426-31
Johnson, D.H.; Kastenberg, W.E.; Griesmeyer, J.M. (1981) Prospectives on the risks of alternative fuel cycles. American Journal of Public Health. 71(9):1050-1057
Koutrakis, P.; Brauer, M.; Briggs, S.L.K. et al. (1991) Indoor exposure to fine aerosols and acid gases. Environmental Health Perspectives. 95:23-28
Kucha, H.; Slupczynski, K.; Prochaska, W. (1993) Health risks and natural gas. Nature. 363:680
Lambert, W.E.; Samet, J.M. (1996) Indoor air pollution. Chapter 48 in: Harber, P.; Schenker, M.B.; Balmes, J.R. (eds). Occupational and environmental respiratory disease. St. Louis, Missouri: Mosby-Year Book, Inc. pp. 784-807
Layton, D.W. and Cederwall, R.T. (1986) Assessing and managing the risks of accidental releases of hazardous gas: a case study of natural gas wells contaminated with hydrogen sulfide. Environmental International 12:519-532
Manahan, S.E. (1991) Toxicological chemistry: a guide to toxic substances in chemistry. Chelsea, Michigan: Lewis Publishers, Inc.
Mills, P.K.; Newell, G.R.; Johnson, D.E. (1984) Testicular cancer associated with employment in agriculture and oil and natural gas extraction. The Lancet. 1(8370): 207-210
Mohan, M.S.; Delgado-Morales, W.; Ilger, J.D.; et al. (1992) Analysis and characterization of arsenic compounds in natural gas. Abstracts of papers of the American Chemical Society. 1992;203:Envr.128
Neely, W.B. (1994) Introduction to chemical exposure and risk assessment. Ann Arbour: Lewis Publishing.
Nigro, P.J.; Bunn, W.B. (1996) Petroleum. Chapter 40 i n: Harber, P.; Schenker, M.B.; Balmes, J.R. (eds). Occupational and environmental respiratory disease. St. Louis, Missouri: Mosby-Year Book, Inc. pp. 688-696
Randolph, T.G. and Moss, R.W. (1981) An alternative approach to allergies. Toronto: Bantam Books
Rea, W.J. (1992) Chemical Sensitivity. Vol. 1. Ann Arbor: Lewis Publishers
Rea, W.J. (1994) Chemical sensitivity: sources of total body load, Vol. 2. Florida: CRC Press
Risser, D.; Schneider, B. (1995) Carbon monoxide-related deaths from 1984 to 1993 in Vienna,
Austria. Journal of Forensic Sciences 1995 :368-371
Rogers, S.A. (1986) The E.I. syndrome: an Rx for environmental illness: are you allergic to the 21st century? Syracuse, NY: Prestige Publishers
Rogge, W.F.; Hildemann, L.M.; Mazurek, M.A. et al. (1993) Sources of fine organic aerosol. 5. Natural gas home appliances. Environ. Sci. Tech. 27:2736-2744
Ross, G.H. (1997) Adverse effects of natural gas (letter). Environmental Health Center, Dallas, Texas
Ross, G.H. (1991) Treatment options in chemical sensitivity. Toxicology and Industrial Health. 8(4):87-94
Rossi, J. III (1996) Sensitization induced by kindling and kindling-related phenomen as a model for Multiple Chemical Sensitivity. Toxicology. 111:87-100
Sax, N.I.; Lewis, R.J. (1987) Hazardous chemicals desk reference. New York, NY: Van Nostrand Reinhold Co.
Schechter, M.T; Spitzer, W.O.; Hutcheon, M.E. et al. (1990) A study of mortality near sour gas refineries in Southwest Alberta: an epidemic unrevealed. Canadian Journal of Public Health. 81:107-113
Schechter, M.T; Spitzer, W.O.; Hutcheon, M.E. et al. (1989) Cancer downwind from sour gas refineries: the perceptions and the reality of an epidemic. Environmental Health Perspectives. 79:283-290
Seinfeld, J.H. (1986) Atmospheric chemistry and physics of air poluution. New York, NY: John Wiley and Sons
Sewell, C.M.; Castle, S.P.; Hull, H.F. et al. (1986) Testicular cancer and employment in agriculture and oil and natural gas extraction. The Lancet 1(8480):553
Sexton, K.; Webber, L.M.; Hayward, S.B. et al. (1986) Characterization of particle composition, organic vapor constituents, and mutagenicity of indoor air pollutant emissions. Environment international. 12:351-362
Tunnicliffe W.S.; Burge, P.S.; Ayres, J.G. (1994) Effect of domestic concentrations of nitrogen dioxide on airway responses to inhaled allergen in asthmatic patients. Lancet. 344:1733-1736
Walsh, P.J.; Dudney, C.S.; Copenhaver, E.D. (1984) Indoor air quality. Florida:CRC Press
Wilson, W.F. (1994) NORM: a guide to naturally occuring radioactive material. Tulsa, Oklahoma: PennWell Publishing Co.
Wojcik, M. (1989) Long-term measurements of Rn and short-lived Rn daughter concentrations in natural gas from distribution line. Health Physics. 57(6):989-991