

Date: May 2002
First off, if it has been a while since you have done chemistry then you should go to the grade 11 material. Go to my main home page at http://www.oocities.org/rjwarren_stm (yellow page) and visit the links listed there.
Look at the topics that are not in this course. This is the material that you should already know. Review it if necessary

 Skip to Units on Enthalpy, Equilibrium and Electrochemistry
Skip to Units on Enthalpy, Equilibrium and Electrochemistry




Hydrogen spectrum is the easiest to study. Three series to know are i) Lyman, ii) Balmer, iii) Paschen. Balmer series is shown below.


Lab on gas discharge tube spectrum; noble gas
To review these spectrum lines visit Spectrum
Zeeman Effect and what it leads to. Below is an actual line spectrum showing the effect. The multiple lines are caused by a strong magnetic field.


Which when solved for the assigned values of l result in these shapes

Electron configurations. Energy level diagrams
Orbital degeneracy, Hund's Rule, Aufbau principle, Pauli Exclusion principle, Heinsburger Uncertainty Principle
How do s, p, d, f fit into the periodic table?

Stability of half and full orbitals, electron configuration exceptions (Cr & Cu). Ionic bonding, electronegativity, ionization energy, electron affinity, polar bond, polar molecules.An example of a polar BOND is shown below


More diagrams of polarity in molecules

Some general overall trends in the periodic table are listed in this diagram

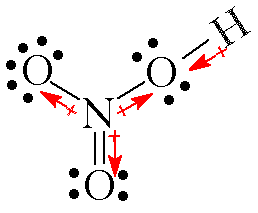
 is an example of nitrogen as an atom
is an example of nitrogen as an atom
Covalent bonding ==> shapes of molecules
VSEPR theory and predicting molecular shapes. See example sheet or student generated note. Similar to what is below but much more complete.


Molecular aggragates
In this chapter or sub-unit we are concerned with NOT what holds atoms together forming molecules, but what forces hold these molecules together with other molecules. If molecules did not stick together all materials would be gaseous. Hence, forces exist that hold molecules together resulting in liquids and when very strong, solids. These different forces will be investigated.
Molecular Architecture & Bonding
Hand-outs on; Language of Chemistry Molecular Architecture, Types of Solids, The Forces: strong & weak, Characteristics of Crystaline solids
Forces holding atoms/molecules together with each other. Key word is INTERMOLECULAR
Bonding Forces: Strong and Weak
Strong => Ionic, Covalent, & Metallic
Weak => Hydrogen bonding, Dipole-dipole, & London or Induced dipole
Molecular forces
London Forces, van der Waals forces, dipole interactions
Metallic, Ionic, Covalent, Network aggragates. Network lattice structures in two and three dimensions.
Properties of these materials



Start out with basic nomenclature, Alkanes, Alkenes Alkynes IUPAC naming system. Cis, trans isomers, naming chloro-alkanes
The alkane, alkene, alkyne family of homologous compounds; some examples are shown below


Preparation and reactions of the following listed series or families. Make sure you can recognize the functional group that defines the family or series .
Functional groups and how to name these compounds.
Simple reactions, tests for unsaturate bonds, formation of esters,

Make sure you know the name of this type of reaction.
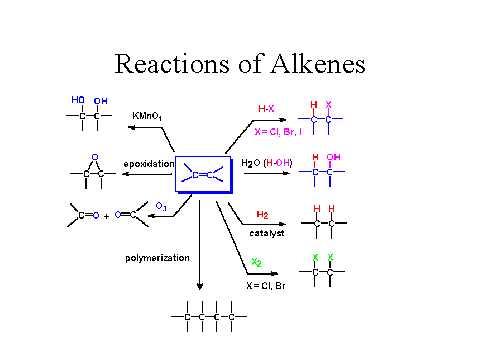
Here's a summary of various reactions for the alkenes
Polymers: Condensation & Addition , names of the six or seven most common, uses and monomers that produce them. Making Nylon 6, 10. Quiz on nomenclature and reactions.
