
![]()

The problem of drug resistance can be attributed primarily to increased selection pressures on P.falciparum in particular, due to indiscriminate and incomplete drug use for self treatment (16). In areas such as Thailand and Vietnam, mosquitoes of the Anopheles dirus and Anopheles minimus species spread the drug resistant parasites. These mosquitoes adapt their biting activity to human behaviour patterns, and maintain intense transmission.
Drug resistant P.falciparum was first reported in Thailand in 1961 (19). Various Plasmodium falciparum 'strains' have now attained resistance to all commonly used and generally available antimalarial drugs (18). In man, the problem of resistance to the common antimalarial drugs such as chloroquine and pyrimethamine, and the decreasing effectiveness of quinine is mainly limited to P.falciparum infection; chloroquine remains the treatment of choice for P.vivax (20). Several mechanisms can account for changes in drug sensitivity in the malaria parasites, for example, physiological adaptations due to non genetic changes, selection of previously existing drug resistant cells from a mixed population under drug pressure, spontaneous mutation, mutation of extranuclear genes, or the existence of plasmid-like factors.
Selection of mutants by the drugs themselves appears an important mechanism (1). In an environment where subtherapeutic levels of the antimalarial drugs are present, those parasites which have resistance through their natural variation or through mutations clearly have an important biological advantage. This means that even though the resistant forms were initially in the minority, the continued drug mediated elimination of intraspecific competition from the non resistant forms has allowed the resistant forms to attain numerical superiority - to the point that drugs such as chloroquine are officially considered useless (20). The majority of studies indicate that drug pressure selection is to blame for the emergence of resistant malaria (1). The subcurative plasma levels of drugs found in many areas where there is uncontrolled and irresponsible prophylaxis and treatment will kill the most drug sensitive forms of the parasite, but select the less sensitive ones- and spontaneous mutations in these forms tends to further reduce the sensitivity of the parasites to the drug (21). Fortunately, the problem of irresponsible prophylaxis has been recognised, and precautions are being taken - for instance, in Zimbabwe it is now illegal to sell chloroquine other than in full courses (30). As drug resistance seems to be genetically determined, gametocytes produced by resistant populations will produce more resistant parasites, promoting spread of the resistant forms.
The Plasmodium parasites have extremely complex genomes, and the ease with which they can switch between the microenvironments in different hosts, and the metabolic changes required illustrates the difficulty in studying the exact modes of action of the antimalarial drugs on parasite metabolism (1). Resistance develops more quickly where a large population of parasites are exposed to drug pressure. The increasingly rapid spread of resistant malaria may be due to an increasingly efficient mosquito vector. This phenomenon may be explained by the increased oocyst formation efficiency that has been observed with resistant species (1). At any rate, the resistant forms undoubtedly have a biological advantage with transmission.
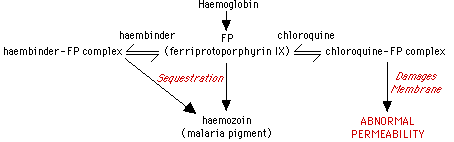
In order to appreciate the physiological nature of resistance, it is necessary to look in more detail at the metabolism of the parasites, and the modes of action of the antimalarial drugs. Intraerythrocytic stages of malaria ingest haemoglobin into food vacuoles. Here exopeptidases and endopeptidases break down haemoglobin into haemozoin (malaria pigment), of which the cytotoxic ferriprotoporphyrin IX is a major component (22). A parasite synthesised binding protein, 'haembinder', seems to sequester the membrane-lytic ferriprotoporphyrin IX into the inert haemozoin complex to protect the Plasmodium membranes from damage. It is now appropriate to discuss a number of antimalarials and apparent adaptations seen in resistance.
Primaquine: This drug has primarily been used against gametocytes and hypnozoites. It has been suggested that the drug works by inhibiting the electron transport chain of the parasite, though, as is so often the case with questions concerning the precise metabolic interactions, this is uncertain. Neither is it certain as to whether it is the drug itself or derived metabolites which have the desired effects (23). There is no evidence that gametocyte resistance exists, but if the drug is used against schizonts, then resistance is rapidly attained (24). The surviving resistant parasites had increased numbers of mitochondria suggesting that the resistance mechanism involves the production of extra organelles to compensate for the damage caused by the drug (1).
Antibiotics: As mentioned in the treatment section, tetracyclines are often used in conjunction with other drugs to combat chloroquine resistant falciparum malaria. Plasmodium protein synthesis appears to be eukaryotic, and is insensitive to chloramphenicol, but affected by cycloheximide. It has been suggested that antibiotics such as tetracycline act on the mitochondrial ribosomes of the parasite, inhibiting protein synthesis. Macrolides such as erythromycin seem to inhibit autophagic vacuole formation, thus potentiating the action of chloroquine (1). Resistance to these compounds is not a current problem.
Sulfonamides: Parasites which become resistant to sulfonamides must bypass the metabolic step at which para-aminobenzoic acid (pABA) is incorporated into dihydropterate. Sulfonamide drugs work by inhibiting pABA, which is needed to synthesise the dihydropterate which is an intermediate compound in the synthesis of tetrahydrofolate. Tetrahydrofolate derivatives serve as donors of one carbon compounds in a variety of essential biosynthetic pathways. Little is known about this side of parasite metabolism, or the exact mechanisms of resistance - though resistance is clearly stable, transmissible, and prolific (1). The resistance seems to be present in all stages of the parasite metabolism. It is possible that gene amplification is the mechanism by which the metabolic block of a pABA inhibitor is overcome.
Proguanil and pyrimethamine (antifols): Both of these compounds inhibit the action of dihydrofolate reductase. As with sulfonides, resistance occurs in all stages of the lifecycle. The dihydrofolate reductase enzymes of resistant strains bind to pyrimethamine 400-800 times less readily than the enzymes of drug sensitive strains (25). Interestingly, high levels of resistance to sulfonamides is associated with hypersensitivity to antifols, and vice versa, so combination treatments have had good effects. Unfortunately, resistance to these drug cocktails is now becoming apparent (1).
Chloroquine and related compounds: It is known that chloroquine mediates its effects on the haemoglobin metabolism of malaria parasites, perhaps preventing the neutralisation of the toxic ferriprotoporphyrin IX. Resistant parasites seem unable to produce haemozoin, but they are still able to digest haemoglobin. In non-resistant forms, most of the ferriprotoporphyrin IX is sequestered in haemozoin, but in the resistant forms, this toxic metabolite seems to become available to the host cell haemoxygenase system for elimination (26). In chloroquine sensitive malaria, the drug is taken up into food vacuoles, and it is proposed that here it competes with the haembinder for the ferriprotoporphyrin IX, to form a destructive compound (28). A diagrammatic representation of chloroquine action is shown below.
 |
Quinine: Quinine and mefloquine cause blebbing of the parasite membranes, and causes aggregations of haemozoin to form. Parasite resistance occurs by uncertain mechanisms, but is stable and transmissible (1).
Artemisnins: These are among the newest and most effective of all antimalarial drugs, and seem to affect protein synthesis. These drugs must be protected, and used rationally, to prevent the emergence of inevitably resistant P.falciparum for as long as possible. In the laboratory, artemisnin resistant forms have already been demonstrated (1).
It is obvious, then, that resistance is an ongoing problem. By 1973, chloroquine was replaced by sulfadoxine-pyrimethamine cocktails, but by 1985, this too was ineffective. Though quinine remains effective, there is a 50% failure rate unless it is supplemented by tetracyclines, and compliance with the 7 day regimen is poor. Between 1985 and 1990, the recommended treatment for malaria in Thailand was mefloquine, combined with sulfadoxine-pyrimethamine at a dose of 15/30/1.5mg/kg body weight, but by 1990 the cure rate had fallen to 71% in adults and 50% in children. This treatment can no longer be used due to resistance (18). The future of chloroquine is not clear, as a recent report (19) suggests that due to the current absence of chloroquine drug pressure, chloroquine sensitivity may well be returning. In this study of patients who were from an area where chloroquine use had long since died out, only one in five of the falciparum infections were truly resistant to chloroquine- presumably due to the lack of selection pressures.
What the future may hold:
During the last three years, the overall number of cases has remained fairly steady at between 2.6 and 2.7 cases reported annually. The real number of cases is likely to be nearer 19 million cases per annum (28). Between 1991 and 1993, 40% of all cases outside of Africa were in India - indicating a serious problem in that country. The percentage of falciparum infections has, however, decreased from 43% in 1991 to 39% in 1993. Urban malaria is a severe problem in India, and 130 towns spread across 17 states are covered by the Urban Malaria Scheme. 80% of all urban malaria cases reported by this scheme were clustered in 15 cities. Drug resistant falciparum here is a spreading problem.Thailand lies in a geographical area which has seen a 20% general decline in overall incidence of malaria between 1992 and 1993. The most intense area of transmission is the border between Thailand and Cambodia, and there are high levels of multidrug resistance in this area, with chloroquine and sulfadoxine-pyrimethamine now considered inadequate. In Thailand, the number of cases has been declining for some time. In 1988, 349,000 cases were recorded, decreasing to 198,000 in 1991 and 152,000 in 1992. In 1993, figures (the most recent available at the time of writing) showed 115,000 cases, 99,000 of which were reported along the borders, in high transmission areas such as the provinces of Trat (bordering Cambodia) and Tak (bordering Myanmar). The drug resistant falciparum strains have clearly spread, and are extremely resistant to chloroquine and sulfadoxine-pyrimethamine. Most recently, Plasmodium falciparum is exhibiting an alarming degree of resistance to mefloquine, and the cure rate with this drug has fallen to below 50% in these sensitive border areas. Trat reported the highest number of cases in the period 1990-1993, 75-80% of which are believed to have been imported from Cambodia.
Imported malaria remains a problem, and studies in the United States, France, and Switzerland indicate that only between 25% and 50% of cases are notified. From these figures, imported malaria cases in Europe are thought to stand at about 16,000 per year. A recent epidemic in Singapore illustrates the severity of importing individuals with current parasitaemia into areas where mosquitoes capable of acting as malaria vectors are to be found. From only 2 infected tourists, 27 other people were rapidly infected. Prompt control measures put a swift end to this outbreak.
So, from the most recent figures available at this time (WHO report from February 1996), the global malaria situation appears to have stabilised, with one or two exceptions such as Turkey, where it remains in decline. However, due to the complication of increasing drug resistance and decreasing therapeutic efficacy of the commonly available drugs, the malaria situation may not be quite so under control as the figures superficially suggest. Though the overall number of cases may not be going up, several factors suggest increasing problems in the future. The unstable political situation across the World, and the recent appearance of so many self declared or 'liberated' countries would suggest that concerted malaria control in these areas is neither going to be a high priority nor practical for some time. It will be interesting to see whether the former Yugoslavia develops a malaria problem, as it is a potentially malarious area which had, by 1969, eliminated endemic malaria within its borders. The current situation there is hardly conducive to control or case notification.
It is clear that imported malaria cases will continue to be a problem for the foreseeable future, as the mobility of tourists and workers is increasing all the time, and non-immune individuals are bound to find themselves at risk. The common prophylactic drugs are, for many areas, obsolete (19), and the use of advanced drugs such as artemisnin derivatives for uncontrolled prophylaxis would be downright irresponsible given the obvious ability of Plasmodium falciparum to attain a high degree of resistance in a short period. It has already been suggested that strains resistant to Artemisnins will appear by the end of the decade (16), and this does seem inevitable.
The form of prophylaxis which may become available soon is a malaria vaccine. Several trials are currently underway, and work has been progressing for several years on this important possibility. Unfortunately, the malaria parasite is not easy for the immune system to deal with via antibodies or cytotoxic T-cells (and humoral response would, presumably, form the basis of any antimalarial vaccine), because it exists within human cells for much of its life cycle - keeping it out of the way of macrophages and antibodies. Within the cell, Plasmodium does not behave like a replicating virus, where MHC Class 2 would enable the cytotoxic T-cells to interdict the replication process, so Plasmodium seems able to evade this defence (1). The sporozoite stages are easy for the immune system of an immunised or immune individual to deal with, but they are transient, and present in such high numbers that some are bound to infect host cells before they are caught. However, a vaccine, though difficult to produce may be possible according to recent clinical trials, and it would certainly be a useful tool.
Drug resistance will certainly be a problem for the future, and it is unlikely that the drug manufacturers will ever produce a drug which can be described as a flawless antimalarial with no possibility of resistance. Plasmodium falciparum has demonstrated that it is extremely good at adapting to any drugs we may use against it- and there is no reason to suspect that this would be different for new compounds. Responsible use of new drugs will be important for the future if artemisnin and whatever follows artemisnin are not to become as clinically compromised as chloroquine.
There are some novel possibilities in the fight against malaria which involve the mosquito vectors. The commercially available, though expensive Bacillus thuringiensis var israelensis H-14 (Bti) may now come into more common use since it can now be grown from coconuts (29). Bti may be introduced into a pond full of mosquito larvae, which ingest these bacilli along with their algae diet. This is their undoing since the bacteria destroy the midgut of the larvae, resulting in considerable mortality. Whether this vector control bio-weapon lives up to its name remains to be seen, but recent reports are promising. Another form of anti-vector measure can be seen in gene therapy to engineer unsuitable vector mosquitoes, as described earlier, and introduce them into the ecosystem in the hope that they may displace the receptive anopheles species and deprive Plasmodium of its vector transmission (1). This sounds like a good idea, but the cost to developing countries would probably be very high as suitable genetically engineered mosquitoes may not be easy to obtain. The theory that these insects will displace the formidable mosquito products of our DDT imposed natural selection also remains to be proven. The genetically engineered mosquitoes would certainly need some drastic biological advantage to achieve this.
In conclusion, after years of decline, the situation is superficially stable, and the numbers of cases may well remain fairly constant. Drug resistance, however, is a menacing threat to malaria control, and, if one thing is certain, Global eradication of malaria is NOT a possibility today!
![]()
Webmaster : Mr. Kulachat Sae-Jang
4436692 MTMT/D
Medical Technology, Mahidol University
E-mail address : oadworld@yahoo.com
