 Product is metal or H2
(g)
Product is metal or H2
(g)VINAY"S CHEMISTRY REVISION NOTES IGCSE 2004 CHEMISTRY
BACK TO MAIN PAGE BACK TO CHAPTER INDEX CONTACT ME: vinz@india.com
Chemistry IGCSE Chapter 5 – Electricity and Chemistry
Electrolysis
· The process of decomposing a compound by passage of an electric current
· When elements are in their simplest form, no further electrolysis possible
· Two electrodes are inserted into an electrolyte and direct current is passed through
· Conductors: all metals and alloys and compound (electrolytic conductors) – a substance that allows electricity to flow through it due to the presence of free electrons
· Insulators/Bad Conductors: opposite
· Electrolytes: ionic compounds which decompose by electrical induction in its dissolved (aqueous) or molten state. (E.g. NaCl(aq), CuSO4 (aq) )
* Electrolyte must contain mobile electrons
· The substance that will be charged and that will be in physical contact with the electrolyte
· Electrolytic Cell: any vessel that can contain electrolyte, allow insertion of electrodes and collection of products. (Current passes through this)
· Anode
o Product is a non-metal or 02 (g)
· Cathode
o The –vely charged electrode (connected to the –ve terminal of battery)
o Attracts cations, which are discharged at the anode
o Reduction (gain of electrons, occur here) – cations gain electrons to become stable
o
 Product is metal or H2
(g)
Product is metal or H2
(g)
· Note: when dealing with electrolytes in molten state, only ions of that substance are present in the electrolyte.
· When ions reach the electrodes they are discharged (lose their charge)
· With molten Lead (II) Bromide this is what happens:
Half Equations:
Cathode: Pb2+ (l) + 2e- à Pb (l)
[the negative electrode gives electrons to the positively charged lead ions so that they become lead atoms]
Anode: Br- (l) à ½ Br2 (l) + e-
[positive electrode takes electrons from bromide ions so that they become diatomic bromine atoms]
·
 Electrons supplied to the anode by
the discharge of bromine ions travel round the external circuit to the cathode.
At the cathode they combine with lead (II) ions.
Electrons supplied to the anode by
the discharge of bromine ions travel round the external circuit to the cathode.
At the cathode they combine with lead (II) ions.
·

Therefore, with molten electrolytes, the metallic element of the compound is discharged at the cathode, and the non-metallic element of the compound is discharged at the anode.
Aqueous Electrolytes
· with these, there is a mixture of the electrolyte and water
· because water is slightly ionizing (ie. Very weak conductor) it forms the ions OH- and H+ ions
· when ionic compounds dissolve in water, the lattice breaks apart and the ions separate and roam freely in the water
· to predict the products formed at each electrode, one must look at the Electrochemical series
· NaCl (aq) = NaCl (s) + H2O (l)
The electrochemical series:
CATIONS (Cathode) ANIONS (Anode)
Ag+ More likely to be chosen OH-
Cu2+ I-
H+ Br-
Pb2+ Cl-
Fe2+ NO3-
Zn2+ SO4-2
Al3+
Mg2+
Na+
Ca2+
K+ Less likely to be chosen
· Less reactive ions are easier to convert back to the metal and therefore have preference
· Nitrates, Sulphates and carbonates are never discharged
· Reactive metals such as sodium and potassium are never discharged (except in the use of mercury electrodes)
Factors that influence discharge
· Location on electro-chemical series
· Concentration of ion (high concentration will discharge easily). However, if gap between anions/cations are large, then the position takes more priority.
· The nature of the electrodes
· In saturated solutions such as brine (OR CONCENTRATED SODIUM CHLORIDE), the Chloride ion concentration is greater than that of Hydroxide ion concentration, so Chloride will be discharged and chlorine evolved instead of oxygen.
· Hydrogen gas is commonly produced at the cathode and oxygen gas at the anode
Some Examples: *bolded is chosen
Zn(NO3)2 AgNO3 (aq)
Anode: NO3-, OH- Anode: OH-, NO3-
Cathode: Zn2+, H+ Cathode: Ag+, H+
Dil. H2SO4 Conc. HCl (aq)
Anode: OH-, SO42- Anode: OH-, Cl-
Cathode: H+, H+ Cathode: H+, H+
· Note: when OH is discharged at the anode (it is a radical, cannot be discharged) therefore it quickly reacts and forms oxygen, which is bubbled off.
Half Equations for OH- and H+ being discharged:
4OH- - 4e à O2 (g) + 2H2O (l)
2H+ + 2e- à H2 (g)
Qty of electricity balanced, 2 multiplied by 2: 4H+ + 4e- à 2H2 (g)
· Although the concentration of hydrogen ions in the solution is very low, it is kept topped up by the ionization of more water molecules.
· At the positive electrode, there are chloride ions and also hydroxide ions
Copper (II) Sulphate Solution and the Purification of Copper
· When carbon (graphite) electrodes are used:
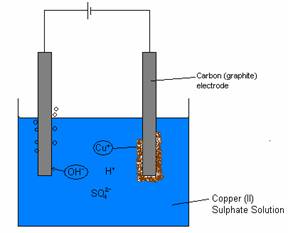
· Copper ions are chosen over H+ and are discharged at the cathode. Copper atoms form on the cathode and Hydroxide is discharge at the anode
(yielding oxygen)
· Therfore, a colourless gas is bubbled off at the anode and the size of the graphite cathode increases
Cu2+(aq) + 2e- à Cu(S)
4OH-(aq) – 4e- à O2(g) + 2H2O(l)
· As the copper ion from the solution decreases, the intensity of the blue in the solution decreases, until a
clear
solution is left.
· When copper electrodes are used:
· Copper atoms from the anode oxidize and form Cu2+ which enters into the solution
· At the cathode, Cu2+ is discharged. The Cu2+ ions from the anode constantly feed the supply on the other end.
· The size of the copper anode decreases whilst copper deposits on the cathode (Note: there is no reaction at the anode)
· Concentration of Cu2+ in the solution does not change ie. colour remains
· The copper deposited on the cathode is PURE

· This method is used in the purification of copper:
o A thin, pure strip of copper is started with at the cathode
o An impure thick strip at the anode is used
o A specific voltage is used, this allows all metals above Cu (on reactivity series) to oxidize (and remain in solution), the rest (to reduce) and fall to bottom of the tank as anode sludge.
Extraction of Aluminium
·
 A powerful method of reduction but
very expensive because large amounts of electricity are used.
A powerful method of reduction but
very expensive because large amounts of electricity are used.
· Aluminum is extracted from its ore, Bauxite (Al2O3)
· The purified bauxite is dissolved in cryolite (to lower the melting point of bauxite and thus reducing costs). Al2O3 is an electrolyte in molten state.
· The ions present are Al3+ and O2-. The Aluminium cations collect at the cathode (and sinks to bottom of tang) where they pick up electrons and aluminium atoms are formed. The oxygen anions give up electrons to form oxygen gas. This immediately reacts with carbon anodes (because of high temps) and produces carbon dioxide.
Manufacture of Chlorine and Sodium Hydroxide from conc NaCl(aq) [Brine]
Note:
· In an aqeous solution, Na will never be discharged. However, the use of mercury electrodes is an exception. It allows Na to dissolve in it and produces Sodium Amalgam.
· The reaction of amalgam with water produces a fine/pure NaOH.
2Na/Hg (l) + 2H2O à 2NaOH (aq) + H2(g) + 2Hg (l)
Electrolysis of Brine
 the
main thing is to make sure that hydrogen and chlorine are kept apart
the
main thing is to make sure that hydrogen and chlorine are kept apart
Electroplating
· The deposition of a metal by electrolysis by “coating” substances with more attractive layers for various reasons
· Objects made from a less expensive metal are given a coating of silver or gold, through a cost-effective technique that makes the metals stick well.
· Layer must be thin
· Coating a cheap metals with a more expensive one.
· Cathode is Coated Metal
· Anode is the material that will cover the cathode
· Used for:
o Decorative purposes
o Protection (prevention of rust by coating with chromium)
o Safety (can drinks are coated with Tin, which is unreactive with juices/drink)
· E.g. Coating a cathode key with pure Nickel anode. Ions of the metal (anode) must be oxidized and enter the solution (Ni(s) à Ni2+ (aq) + 2e-) ß entering solution
· There is usually no reaction at the anode, nothing is yielded.
· In ‘galvanised’ iron, a layer of zinc is coated
