|
|
Reaction Rates
There are many factors that can influence the speed at which a
reaction takes place, and these can change with time. In
general, it is a function of the state of the system and the nature of the reactants themselves. It is
reasonable to assume that, for a reaction to occur, the molecules
of the reactants must collide and collide with sufficient energy to initiate a reaction. The number of collisions per
unit time can be expected to change and increase with the number of molecules
of the reactants available. At higher temperatures the
number of collisions is also expected to increase (the molecules
are more agitated) and collide with more energy. The rate of reaction also depends on the
presence and amount of catalysts or inhibitors. These
materials increase or decrease the reaction speed, respectively,
although they don't appear in the chemical equation.m (They may be found written above the arrow).
The following note presents some of the factors that result in a change in a Reaction Rate
Concentration
-
As the concentration of the reactants increases, the reaction rate
increases.
-
WHY?
-
According to the collision theory, the rate of a reaction is directly
proportional to the number of effective collisions per second
between the reactant molecules.
-
What is an Effective Collisions? - the fraction of total collisions that actually result
in the formation of the product(s).
-
If the concentration of the reactants increases the greater the number of total collisions that can occur.
-
The greater the frequency of total collisions, the greater the frequency
of effective collisions.
-
If the frequency of effective collisions increases, so does the reaction
rate which results in more product being formed.
Surface Area
-
As the surface area of the reactants increases, the reaction rate
increases.
-
WHY?
-
Increasing the surface area of the reactants results in a higher number of
reaction sites
-
What is a Reaction sites? - specific sites on molecules at which reactions occur. More molecules are exposed to
collisions with other reacting molecules.
-
Increasing the number of reaction sites increases the number of total collisions.
-
The greater the frequency of total collisions, the greater the frequency
of effective collisions.
-
If the frequency of effective collisions increases, so does the reaction
rate.
Imagine a reaction between magnesium metal and a dilute acid like hydrochloric acid. The reaction involves collision between magnesium atoms and hydrogen ions.
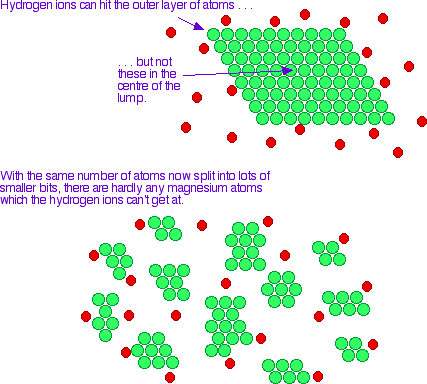 Increasing the number of collisions per second increases the rate of reaction.
Increasing the number of collisions per second increases the rate of reaction.
Temperature
-
As the temperature of a system increases, the reaction rate
increases.
-
WHY?
-
What is Temperature (T)? - A measure of the average kinetic energy
(KEavg) of the reacting particles of a substance.
-
Increasing T increases KEavg of the molecules in the reaction mixture..
-
At higher T, the fraction of molecules with energies which enable the reaction to proceed
increases. All chemical reactions require energy to initiate the process of reaction. If molecules do not have
this specific amount of energy, then no reaction will occur. That why giving the reacting molecules more energy
will increase the number of effective reaction collisions.
Catalysts
-
Examples of common catalysts:
-
Platinum
-
Nickel
-
Manganese Dioxide (MnO2)
When a chemical reaction happens, we say that the
reactants react
to form the products
e.g.
HCl |
+ |
NaOH |
 |
NaCl |
+ |
H2O |
Reactant A |
|
Reactant B |
|
Product C |
|
Product D |
hydrogen chloride |
|
sodium hydroxide |
|
sodium chloride |
|
water |
So, to alter the rate of a reaction, we need to do
something to the reactants. There are 4 main ways to change the rate of a
chemical reaction. Remember, an increase
in rate
means it is going
faster:
Here's a small summary
1 |
Change the
concentration
of the reactants (or the pressure, if the reactants are gases). |
Increasing the
concentration
of reactants (making them stronger),
increases
the
rate. |
2 |
Change the
temperature
of the reactants. |
Increasing the
temperature
of reactants, increases
the
rate. |
3 |
Change the
size of
the solid lumps of a
reactant. |
Using smaller
lumps
(increasing the surface area),
increases
the
rate. |
4 |
Use a
catalyst. |
Using a
catalyst,
usually increases
the rate
(some catalysts can decrease the
rate). |
If you had to prove any of these conclusions,
what experiments could be conducted and how would you know
if the rate has changed?
For an additional note courtisey of Dallas Community College Click Here


