

In this note you will learn how to name compounds given tht formula and the reverse. You have the formula and you write the name. Then I shall deal with polyatomic ions and how to use them in names and formula.
Univalent elements are those metal elements that have only one allowed valence number. Some are found in column I & II on the periodic chart. Zinc, aluminum, silver, and cadmium also come to mind. Being metals the valence number represents the number of electrons that the elemnet will lose.
Lost electrons must go somewhere, they go to the nonmetals which wish to fill their outer shell.
Everything listed above holds true in this case. There is just and extra step. These cations have more than one valence value. You simply have to say what it is or use the value given to you.
Iron has two valencies +2 and +3 so iron can form two different compounds, one with the +2 valence and the other with the +3 valence.
When dealing with divalent cations there is always three steps to either writting the name or the formula.
When writing the name given the formula follow these steps:
Naming and Writing Formula of chmical compounds
This is a taught topic with board notes.
 Two practice activities with answers provided
are to be done at home with a subsequent work sheet of this activity for the next day.
Two practice activities with answers provided
are to be done at home with a subsequent work sheet of this activity for the next day.If you think about this example everything should be obvious. Again, Examples are done in class.
Listed below is a small chart showing names and formula of several compound. When reading these examples think how the name corresponds to the formula and visa versa.
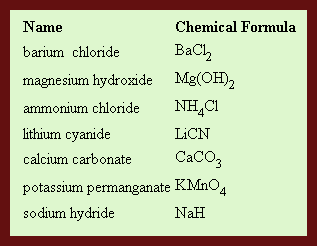
What need here is practice; several worksheets to be given out. Just check the title with the answer sheet.







 Try the example then move mouse over the question to see the answer. Make sure you understand the answer and if you don't then ask for an explination.
Try the example then move mouse over the question to see the answer. Make sure you understand the answer and if you don't then ask for an explination.