 Acids
and Alkalis
Acids
and Alkalis Acids
and Alkalis
Acids
and Alkalis
Most people know that acids are caustic – that is they burn us. Fewer know why this occurs.
It all has to do with hydrogen – water is made of two hydrogen atoms and one oxygen atom stuck together as above. This is why water is known as H2O.
Very rarely, one of the hydrogen molecules ‘falls off’ – this individual hydrogen, for reasons we shall not go into, is very reactive and can damage other molecules. Luckily it does not remain detached for long (fractions of a second) and soon re-attaches to form the water molecule again. An acid is something that, added to water, effectively increases the rate at which hydrogen atoms ‘fall off’ of water molecules – this in turn means there are more of the reactive hydrogen atoms in the water at once and so more damage is caused to anything in contact with them – this is why acids are corrosive.
If the above information is not easily understood, then do not worry. It is interesting background but not necessary to understand all of the following.
What is necessary is an understanding of pH. This is the unit used for measuring acidity.
|
pH
1 |
pH
3 |
pH
5 |
pH
7 |
pH
10 |
|
Very Strong Acid |
Strong Acid |
River Water |
Neutral |
Alkali |
An alkali is the opposite of an acid – it is caused by an excess of the part of the water molecule that is left when one of the hydrogen atoms ‘falls off’. Like strong acids, strong alkalis are corrosive and may dissolve living things.
It is important because there are many acidic environments and a few alkali ones on earth that bacteria live within. In fact, some of the reactions that bacteria carry out in their lives actually make their environment acidic. A good example of this is the sulphur-eating bacteria mentioned earlier. When these bacteria consume sulphur, a by-product of the reaction is sulphuric acid (a very strong acid). If the bacteria were not resistant to this then their own products would very rapidly kill them.
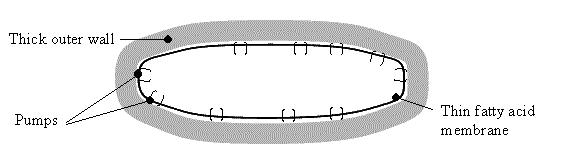
The answer lies in a clever pump system on the thin inner membrane (see figure below). The outer thick wall is very porous and identical to that of non-acid resistant bacteria (it is naturally acid resistant). It allows water and dissolved molecules free entry but the inner, fatty acid membrane can be selective as it has pumps all along its surface that specifically pump desirable things in, and undesirable things out. This means that enough of the lone hydrogen atoms can be pumped out after entry to allow the inside of the bacteria to be at a healthy pH of 6-7, even though the outside may be as low as pH1
As with so many of the things discussed, it is emphasised that not all bacteria are capable of doing this and to differing degrees. Bacteria that survive as low as pH 1 are much less common than those surviving to pH 3.