Pauling and Sickle cell anaemia
TOP
Full Page List
The source of the following transcript forms part of the Ava
Helen and Linus
Pauling Papers at: Oregon State University Libraries where it may be viewed in image
form. Please visit this
site which has much other material besides.
Transcript of a reprint from Science, November 25, 1949. Vol.
110; No. 2865,
Pages 543-548.
Prepared by M. Kudrati MB BCh (Cantab)
Sickle Cell Anaemia, a Molecular disease
Linus Pauling, Harvey A. Itano, S.J.Singer, and Ibert C.
Wells
Gates and Crellin Laboratories of Chemistry,
California Institute of Technology, Pasadena, California.
The erythrocytes of certain individuals possess the capacity to
undergo
reversible changes in shape in response to changes in the partial
pressure of
oxygen. When the oxygen pressure is lowered, these cells change
their forms from
the normal biconcave disc to crescent, holly wreath, and other
forms. This process
is known as sickling. About 8 percent of American Negroes
possess this
characteristic; usually they exhibit no pathological consequences
ascribable to it.
This people are said to have sicklemia, or sickle cell trait.
However, about 1 in 40
(4) of these individuals whose cells are capable of sickling suffer
from a severe
chronic anemia resulting from excessive destruction of their
erythrocytes; the term
sickle cell anaemia is applied to their condition.
The main observable difference between the erythrocytes of
sickle cell trait and
sickle cell anemia has been that a considerably greater reduction
in the partial
pressure of oxygen is required for a major fraction of the trait cells to
sickle than for
the anemia cells (11). Tests in vivo have demonstrated that
between 30 and 60
percent of erythrocytes in the venous circulation of sickle cell
anemic individuals,
but less than 1 percent of those in the venous circulation of
sicklemic individuals,
are normally sickled. Experiments in vitro indicate that under
sufficiently low oxygen
pressure, however, all the cells of both types assume the sickled
form.
The evidence available at the time that our investigation was
begun indicated
that the process of sickling might be intimately associated with the
state and the
nature of the of the hemoglobin within the erythrocyte. Sickle cell
erythrocytes in
which the hemoglobin is combined with oxygen or carbon
monoxide have the
biconcave disc contour and are indistinguishable in that form from
normal
erythrocytes. In this condition they are termed promeniscocytes.
The hemoglobin
appears to be uniformly distributed and randomly oriented within
normal cells and
promeniscocytes, and no birefringence is observed. Both types of
cells are very
flexible. If the oxygen or carbon monoxide is removed, however,
transforming the
hemoglobin into the uncombined state, the promeniscocytes
undergo sickling. The
hemoglobin within the sickled cell appears to aggregate into one
or more foci, and
the cell membranes collapse. The cells become birefringent (11)
and quite rigid.
The addition of oxygen or carbon monoxide to these cells
reverses these
phenomena. Thus the physical effects just described, depend on
the state of
combination of the hemoglobin, and only secondarily, if at all, on
the cell
membrane. This conclusion is supported by the observation that
sickle cells when
lysed with water produce discoidal, rather than sickle-shaped,
ghosts (10).
EXPERIMENTAL METHODS
The experimental work reported in this paper deals largely
with an
electrophoretic study of these hemoglobins. In the first phase of the
investigation,
which concerned the comparison of normal and sickle cell anemia
hemoglobins,
three types of experiments were performed : 1) with
carboxyhemoglobins; 2) with
uncombined ferrohemoglobins in the presence of dithionite ion, to
prevent oxidation
to methemoglobins; and 3) with carboxyhemoglobins in the
presence of dithionite
ion. The experiments of type 3 were performed and compared with
those of type 1
in order to ascertain whether the dithionite ion itself causes any
specific
electrophoretic effect.
The experimental work reported in this paper deals largely
with an
electrophoretic study of these hemoglobins. In the first phase of the
investigation,
which concerned the comparison of normal and sickle cell anemia
hemoglobins,
three types of experiments were performed : 1) with
carboxyhemoglobins; 2) with
uncombined ferrohemoglobins in the presence of dithionite ion, to
prevent oxidation
to methemoglobins; and 3) with carboxyhemoglobins in the
presence of dithionite
ion. The experiments of type 3 were performed and compared with
those of type 1
in order to ascertain whether the dithionite ion itself causes any
specific
electrophoretic effect.
Samples of blood were obtained from sickle cell anemic
individuals who had
not been transfused within three months prior to the time of
sampling. Stroma-free
concentrated solutions of human adult hemoglobin were prepared
by the method
used by Drabkin (3). These solutions were diluted just before use
with the
appropriate buffer until the hemoglobin concentrations were close
to 0.5 grams per
100 milliliters, and then were dialyzed against large volumes of
these buffers for 12
to 24 hours at 4o C. The buffers for the experiments of
types 2 and 3
were prepared by adding 300 ml of 0.1 ionic strength sodium
dithionite solution to
3.5 liters of 0.1 ionic strength buffer. About 100 ml of 0.1 molar
NaOH was then
added to bring the pH of the buffer back to its original value.
Ferrohemoglobin
solutions were prepared by diluting the concentrated solutions
with the
dithionite-containing buffer and dialyzing against it under a nitrogen
atmosphere.
The hemoglobin solutions for the experiments of type 3 were
made up similarly,
except that they were saturated with carbon monoxide after dilution
and were
dialyzed under a carbon monoxide atmosphere. The dialysis
bags were kept in
continuous motion in the buffers by means of a stirrer with a
mercury seal to prevent
the escape of the nitrogen and carbon monoxide gases.
The experiments were carried out in the modified Tiselius
electrophoretic
apparatus described by Swingle (14). Potential gradients of 4.8 to
8.4 volts per
centimetre were employed, and the duration of the runs varied
from 6 to 20 hours.
The pH values of the buffers were measured after dialysis on
samples which had
come to room temperature.
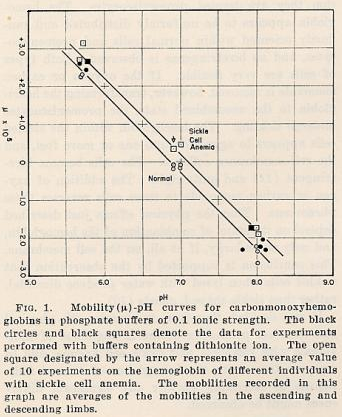
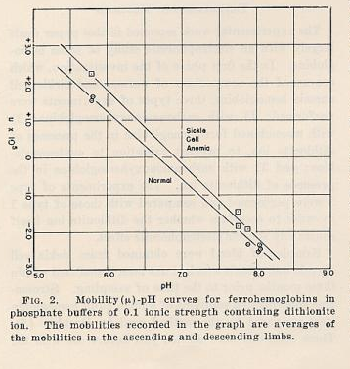
RESULTS
The results indicate that a significant difference exists between
the
electophoretic mobilities of hemoglobin derived from erythrocytes
of normal
individuals and from those of sickle cell anemic individuals. The
two compounds
are particularly easily distinguished as the carbonmonoxy
compounds at pH 6.9 in
phosphate buffer of 0.1 ionic strength. In this buffer the sickle cell
anemia
carbomonoxyhemoglobin moves as a positive ion, while the
normal compound
moves as a negative ion, and there is no detectable amount of
one type present in
the other1. The hemoglobin derived from
erythrocytes of individuals
with sicklemia, however, appears to be a mixture of the normal
hemoglobin and sickle cell anemia hemoglobin in roughly equal proportions. Up to
the present time
the hemoglobins of 15 persons with sickle cell anemia, 8 persons
with sicklemia,
and 7 normal adults have been examined. The hemoglobins of
normal adult white
and negro individuals were found to be indistinguishable.
The mobility data obtained in phosphate buffers of 0.1 ionic
strength and
various values of pH are summarised in Figs. 1 and 2.
2
Mobility curves
| Fig.1 |
Fig.2 |
|
|
|
Notes:
1Occasionally small amounts(less than 5 percent
of the total
protein) of material with mobilities different from that of either kind of
hemoglobin
were observed in these uncrystallized hemoglobin preparations.
According to the
observations of Stern, Reiner and Silber (12) a small amount of a
component with a
mobility smaller than that of oxyhemoglobin is present in human
erythrocyte
hemolyzates.
2The results obtained with carboxyhaemoglobins
with and without
dithionite ion in the buffers indicate that the dithionite ion plays no
significant role in
the electrophoretic properties of the proteins. It is therefore of
interest that
ferrohemoglobin was found to have a lower isoelectric point in
phosphate buffer
than carbonmonoxyhemoglobin. Titration studies have indicated
that (5,6) that
oxyhemoglobin (similar in electrophoretic properties to the
carbonmonoxy
compound) has a lower isoelectric point in....
(Text of article ends here and the source for this article leaves
out the last note
together with the list of references quoted in the text)
The source of the following additional transcript forms part of
the Ava Helen and
Linus Pauling Papers at:
Oregon State University Libraries. Please visit this site which has much
other material
besides.The speech notes indicate his early interest in
haemoglobin, and offers a
glimpse into his ordinary self.
Manuscript: Hemoglobin and Magnetism, formal chapter
installation of Sigma
Xi, Oregon State College, Corvallis, Oregon. May 12, 1937. Page
01
Handwritten address for a speech at a banquet
Author: Linus Pauling
Talk after installation of Sigma Xi, Corvallis.
Hemoglobin and Magnetism
I am happy to be here on this occasion.
When Professor Graf
wrote to me that Professors Milne and Simmons and their
committee asked me to
come, I was strongly tempted to accept, even though my better self
told me that I
should refuse. I don't mind lecturing to students or at scientific
meetings, but a
banquet is something different; talks at banquets should be left to
easy talkers -
historians, lawyers, economists, philosophers. In this case
however, the tempter
won - I decided that I would have the pleasure of coming here
even though the
audience had to suffer for it.
The general subject that I shall talk about
under the disguise
of the title "Hemoglobin and Magnetism", is a new branch of
chemistry, modern
structural chemistry. This subject involves the determination of the
structures of
molecules - the exact location of the atoms in space relative to one
another - and
the interpretation of the chemical and physical properties of
substances in terms of
the structure of their molecules. The new information about the
structure of
molecules is detailed and accurate and goes far beyond the
structural formulas of
the organic chemist in the same way that the final architectural
drawings of a
building go beyond a preliminary rough sketch. Modern structural
chemistry is a
new subject - twenty years ago detailed structural information was
available for only
half-dozen molecules, and ten years ago for only a few dozen.
Now scores of new
molecules are being studied every year, mainly by the physical
methods of
spectroscopy, x-ray and electron diffraction, measurement of
magnetic properties
etc., and the results obtained have been found to be useful for the
biochemist
studying vitamins as well as the inorganic chemist studying
complex inorganic
substances (polychromates deleted in original text).
(next paragraph deleted)
With the subject so new, its story is of course
short; without
doubt there would be much more to talk about after another
decade of
development, and I feel like the little girl in the story, that I would
have been better to
have waited a while. A fond father said to his little girl, "Darling, a
man has offered to
give us a million dollars if we will sell little brother to him; just
imagine what we could
do with all that money - you could have anything you wanted. Shall
we sell him?"
The little girl said, "No, don't sell him"; but then, while the father
was beaming at this
display of high-mindedness on her part, she continued, saying,
"Let's keep him till
he's a little bigger, and he'll be worth more."
As a single substance to use as an example
of the
application of the methods of modern structural chemistry I have
selected
hemoglobin; with particular emphasis on its magnetic
properties.
Back to Top
Full Page Index
Email!
| Prepared for the
Internet, © M. E. Kudrati, 2006:This document may be
reproduced and redistributed, but only in its entirety and with full
acknowledgement of its source and
authorship
|

