Summary
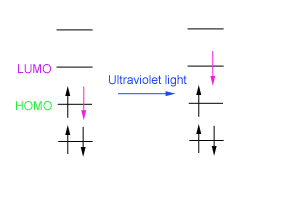
Ultraviolet-visible (UV-vis) radiation has the property of being able to promote an electron to a higher energy level.
The electron is promoted from the HOMO (highest occupied molecular orbital) to the LUMO (lowest unoccupied molecular orbital).
This electronic transition occurs at a particular wavelength in the UV-vis region of the electromagnetic spectrum. At that wavelength, radiation is absorbed by the sample and this is detected by the spectrometer.
The Beer-Lambert law is used to relate absorption of UV-vis radiation to the concentration of the substance, the path length of the cell and the molar absorption coefficient. The molar absorption coefficient is a constant for every substance and is a measure of the amount of radiation absorbed per unit concentration of a substance.
You could now either go on to do the quiz or go straight on to the next section which is about the opposite of absorption spectroscopy and is known as fluorescence.