|
|
Molten Carbonate
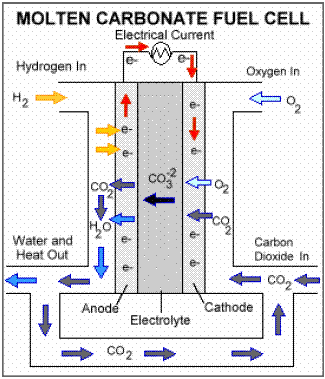
Molten carbonate fuel cells (MCFCs)
are currently being developed for natural gas and coalbased power plants
for electrical utility, industrial, and military applications. MCFCs are
hightemperature fuel cells that use an electrolyte composed of a molten
carbonate salt mixture suspended in a porous, chemically inert ceramic
lithium aluminum oxide (LiAlO2) matrix. Since
they operate at extremely high temperatures of 650ºC (roughly 1,200ºF)
and above, non-precious metals can be used as catalysts at the anode and
cathode, reducing costs. Improved efficiency is another reason MCFCs
offer significant cost reductions over phosphoric acid fuel cells (PAFCs).
Molten carbonate fuel cells can reach efficiencies approaching 60
percent, considerably higher than the 37-42 percent efficiencies of a
phosphoric acid fuel cell plant. When the waste heat is captured and
used, overall fuel efficiencies can be as high as 85
percent.
Unlike alkaline, phosphoric acid, and polymer electrolyte membrane fuel
cells, MCFCs don't require an external reformer to convert more
energy-dense fuels to hydrogen. Due to the high temperatures at which
they operate, these fuels are converted to hydrogen within the fuel cell
itself by a process called internal reforming, which also reduces cost.
Molten carbonate fuel cells are not prone to carbon monoxide or carbon
dioxide "poisoning" they can even use carbon oxides as fuel making them
more attractive for fueling with gases made from coal. Although they are
more resistant to impurities than other fuel cell types,
scientists are looking for ways to make MCFCs resistant enough to
impurities from coal, such as sulfur and particulates.
The primary disadvantage of current MCFC technology is durability. The
high temperatures at which these cells operate and the corrosive
electrolyte used
accelerate component breakdown and corrosion, decreasing cell life.
Scientists are currently exploring corrosion-resistant materials for
components as well as fuel cell designs that increase cell life without
decreasing performance.
Solid Oxide
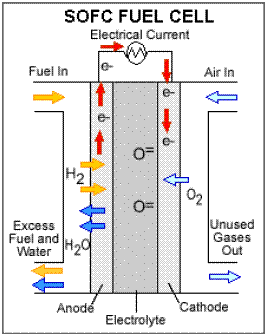
Solid oxide fuel cells (SOFCs) use a
hard, non-porous ceramic compound as the electrolyte. Since the
electrolyte is a solid, the cells do not have to be constructed in the
plate-like configuration typical of other fuel cell types. SOFCs are
expected to be around 50-60 percent efficient at converting fuel to
electricity. In applications designed to capture and utilize the
system's waste heat (co-generation), overall fuel use efficiencies could
top 80-85 percent. Solid oxide fuel cells operate at very high
temperatures around 1,000ºC (1,830ºF). High
temperature operation removes the need for precious-metal catalyst,
thereby reducing cost. It also allows SOFCs to reform fuels internally,
which enables the use of a variety of fuels and reduces the cost
associated with adding a reformer to the system. SOFCs are also the most
sulfur-resistant fuel cell type; they can tolerate several orders of
magnitude more sulfur than other cell types. In addition, they are not
poisoned by carbon monoxide (CO), which can even be used as fuel. This
allows SOFCs to use gases made from coal.
High-temperature operation has
disadvantages. It results in a slow startup and requires significant
thermal shielding to retain heat and protect personnel, which may be
acceptable for utility applications but not for transportation and small
portable applications. The high operating temperatures also place
stringent durability requirements on materials. The development of
lowcost
materials with high durability at cell operating temperatures is the key
technical challenge facing this technology.
Scientists are currently exploring
the potential for developing lower-temperature SOFCs operating at or
below 800ºC that have fewer durability problems and cost less. Lower
temperature SOFCs produce less electrical power, however, and stack
materials that will function in this lower temperature range have not
been identified.
Regenerative
(Reversible) Fuel Cells
Regenerative fuel cells produce
electricity from hydrogen and oxygen and generate heat and water as
byproducts, just like other fuel cells. However, regenerative fuel cell
systems can also use electricity from solar power or some other source
to divide the excess water into oxygen and hydrogen fuel this process is
called "electrolysis."
This is a comparatively young fuel cell technology being developed by
NASA and others.
|