Cyclodextrins
An Overview
Intensive Program
Agriculture: Source of Raw Materials for Industry
Gent 2001 ©
Matthias Arenskötter, Westfälische Wilhelms-Universität, Münster, Germany
Florence Folmer, University of Wales Bangor, UK
Chris Llewellyn, University of Wales Swansea, UK
Aurélie Pardo, Institue Nationale Polytechnique de Toulouse, France
Frank Reinecke, Westfälische Wilhelms-Universität, Münster, Germany
Grazia Trebbi, Universita´ di Bologna, Italy
Introduction
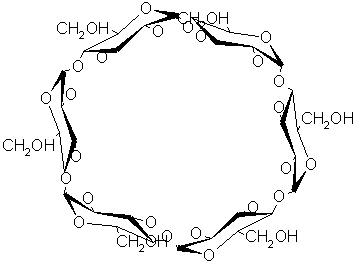
Cyclodextrins (CDs) are torus shaped cyclic oligomers consisting of 6 (a),7 (b) or 8 (g-CD) glucose units with a-1,4-linkages with a hydrophobic cavity and a hydrophilic exterior. In the pharmaceutical industry, Cyclodextrins have mainly been used as complexing agents to increase the aqueous solubility of poorly water-soluble drugs, and to increase their bio- availablity and stability . In addition, Cyclodextrins can be used to reduce or prevent gastro-intestinal (GI) or ocular irritation, reduce or eliminate unpleasant smells or tastes, prevent drug-drug or drug-additive interactions, or even to convert oils & liquid drugs into micro-crystalline or amorphous powders. The ability of Cyclodextrins to form complexes with a wide variety of organic compounds , helps to alter the apparent solubility of the molecule, to increase the stability of compound in the presence of light , heat and oxidizing conditions and to decrease volatility of compound. Cyclodextrins can also be used as processing aids to isolate compound from natural sources and to remove unwanted compounds such as cholesterol from food products.
Cyclodextrins can be used as process aids to remove specific components from a mixture or minerals. The components which have been removed may either be discarded or further purified and the Cyclodextrin recycled. Heat method, High pH method, or solvent method can be used for the separation. Cyclodextrins are capable of forming of inclusion complexes.
Cyclodextrins are produced by a highly selective enzymatic synthesis consisting of Six, Seven, or Eight glucose monomers averaged in a donut shaped ring, which are denoted alpha, beta or gamma Cyclodextrin respectively. The specific coupling of the glucose monomers gives the Cyclodextrin a rigid conical molecular structure with a hollow interior of a specific volume.

Fig. 1: a-Cyclodextrin.
History
Cyclodextrins (CD) were discovered 1891 by Villier [1]. Schardinger described their properties in 1903. Tilden and Hudson were able to show that the conversion of starch to CD was due to the action of an enzyme, CD Glycosyl-Transferase (CGTase), which was secreted into the culture medium by Bacillus macerans grown on starch (1939). Since 1957 the complexation ability of CDs have widely been accepted (Cramer, French) [2]. In 1981 the first International Symposium on CDs was held. Ogawa [3] performed total synthesis (1987), followed by the synthesis of cyclo[D-Glcp(1->4)]5 (1994). Today CDs are only synthesized either by fermentation or enzymatically. Many CGTases from different microorganisms are known, cloned, sequenced, characterized and used for production of CDs.
Applications
Because of their unique properties CDs allow „packaging on a molecular level" of various molecules which is applied in pharmaceutics, food and flavours etc...
Used in chromatographic columns CDs can separate stereoisomers.
Derivatives of CDs can even be used to mimic enzymes.
Microbial Production and Purification
CGTases are produced by many different bacterial species of the genus Bacillus but also by Flavobacterium sp., Klebsiella pneumoniae and Micrococcus sp. Genes have been cloned and recombinantly expressed in Escherichia coli and other organisms, including for example Solanum tuberosum (Potato) [4]. The CGTase acts on linear starch and transfers a part of the chain to its own non-reducing end. Enzymatic synthesized CDs are selectively precipitated by organic solvents (see Table 1).
Table 1:
|
Precipitation Agent |
Yield (%) |
|
|
a -Cyclodextrin |
1-decanol |
40 |
|
b -Cyclodextrin |
toluene |
50-60 |
|
g -Cyclodextrin |
cyclohexadec-8-en-1-ol |
40-50 |
Properties
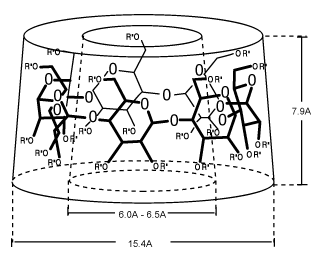 CDs have hydrophobic cavities of different sizes enabling the complexation of hydrophic guest molecules. These complexes represent a solution for insolubility. They have neither a reducing nor a non-reducing end-group. Cyclodextrins form a rigid conical molecular structure with a hollow interior of a specific volume (Table 2, Fig. 2). This internal cavity, which is hydrophobic in its nature, is a key structural feature of the Cyclodextrins, providing the ability to complex and contain a variety of „guest" molecules (e.g. aromatic, alcohol, halides and hydrogen halides, fatty acids and other esters etc.). The guests must satisfy the size criterion of fitting at least partially into the Cyclodextrin internal cavity, resulting in an inclusion complex.
CDs have hydrophobic cavities of different sizes enabling the complexation of hydrophic guest molecules. These complexes represent a solution for insolubility. They have neither a reducing nor a non-reducing end-group. Cyclodextrins form a rigid conical molecular structure with a hollow interior of a specific volume (Table 2, Fig. 2). This internal cavity, which is hydrophobic in its nature, is a key structural feature of the Cyclodextrins, providing the ability to complex and contain a variety of „guest" molecules (e.g. aromatic, alcohol, halides and hydrogen halides, fatty acids and other esters etc.). The guests must satisfy the size criterion of fitting at least partially into the Cyclodextrin internal cavity, resulting in an inclusion complex.
Fig. 2: Model of the torus shape formed by a Cyclodextrin.
Tab. 2: Important Properties of Cyclodextrins.
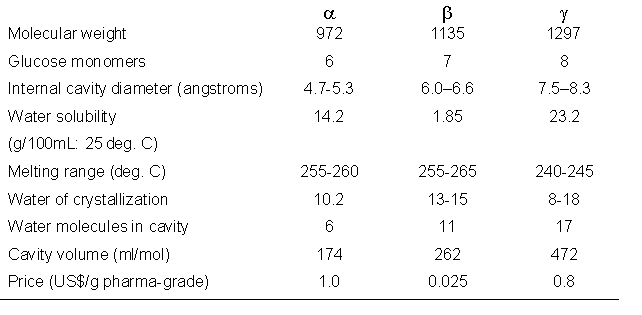 Stability
Stability
CDs are not decomposed by hot aqueous alkali and rather resistant to acid hydrolysis.
CDs are resistant to a-amylases (except microbial enzymes) and they are completely resistant to yeast-fermentation and b-amylases.
Applications
Cyclodextrins have many applications particularly in pharmaceuticals [5], in foods, in cosmetics and in chemical industry and etc… .
The major advantages of these applications are as follows:
1. Pharmaceuticals
By the properties, the cyclodextrins represent a solution for insolubility. Indeed, the hydrophobic cavity provides a favorable environment in which the drug molecule is held in place.This association isolates the drug from the aqueous solvent and may increase the drug’s water solubility and stability. In particular, a drug substance has to have a certain level of water solubility to be readily delivered to the cellular membrane but it needs to be hydrophobic enough to cross the membrane.
The majority of pharmaceutical active agents do not have sufficient solubility in water and traditional fomulations systems for insoluble drugs involve a combination of organic solvents, surfuctants and extreme pH conditions which often cause irritation or other adverse reactions. Cyclodextrins are not irritant and offer distinct advantages such as the stabilization of active compounds, reduction in volatility of drug molecules, and masking of malodours and bitter tastes.
2. Foods and Flavours
Cyclodextrins are used in food formulations for flavour protection and flavour delivery. Artificial flavours are volatile oils or liquids and complexation with cyclodextrins provide a promising alternative to the conventional encapsulation technologies used for flavour protection. Cyclodextrins are also used as process aids to remove cholesterol from products such as milk, butter and eggs.
3. Cosmetics
The major benefits of cyclodextrins in this sector are stabilization, odour control and improvment upon conversion of a liquid ingredient to a solid form. Applications include toothpaste, skin creams, liquid and solid fabric softeners, paper towels, tissues and underarm shields.
4. Chemical industry
In the chemical industry, cyclodextrins are widely used to catalyse reactions by CD-derivates used as artificial enzymes (Fig. 3).
Fig. 3: Hydrolysis of a phosphate diester by a catalyst containing two cyclodextrins [6].
Moreover, they are used to separate isomers and enantiomers [7], to aid in various processes and to remove or detoxify waste materials.
In fact, cyclodextrin polymers have been used for the recovery of aromatics pollutants like phenols, naphthols, or polychlorophenols which may be found in industrial waste water.
5. Agricultural industry
Cyclodextrins form complexes with a wide variety of agricultural chemicals including herbicides, insecticides, fungicides, repellents, pheromones and growth regulators.
Benefits include stabilisation and increased solubility.
6. Adhesives, coatings and other polymers
Cyclodextrins increase the tackness and adhesion of some hot melts and adhesives. They also make additives and blowing agents compatible with hot melt systems. The interaction between polymer molecules in associative thinckening emulsion-type coastings such as paints tends to increase viscosity and cyclodextrins can be used to counteract this undesirable effect.
References
Internet
Articles
[1] A. Villiers, Compt. Rend. Acad. Sci. Paris 112 (1891) 536.
[2] F. Cramer, F. M. Henglein, Chem. Ber. 90 (1957) 2561.
[3] Y. Takahashi, T. Ogawa, Carbohydr. Res. 164 (1987) 277.
[4] JV Oakes, Shewmaker CK, Stalker DM, Biotechnology (N Y) 1991 Oct;9 (10):982-6: „Production of cyclodextrins, a novel carbohydrate, in the tubers of transgenic potato plants."
[5] E. Schneidermann, Stalcup AM. J Chromatogr B Biomed Sci Appl 2000 Aug 4;745(1):83-102 „Cyclodextrins: a versatile tool in separation science"
[6] R. Breslow, Acc. Chem. Res. (1995), 28, 146-153: „Biomimetic Chemistry and Artificial Enzymes: Catalyst by Design".
[7] J. Szejti, Med Res Rev 1994 May;14(3):353-86: „Medicinal applications of cyclodextrins"