7.2 PHASE II: CLINICAL TRIAL OF SAMBONG TABLET AS DIURETIC
Clinical Design: Randomized, comparative, parallel, positive control (thiazide)
among in-patients with edema.
Patient inclusion: 1) Patients aged 20-75 years. With edema secondary to congestive heart failure (CHF Class I-II) and/or mild hypertension with altered fluid and electrolyte states.
7.2.1 Definition:
Mild
hypertension –BP= 140-160 / 90-105 mmHg
(after 3 determinations sitting position)
CHF Class I – symptomatic only with greater than ordinary activity.
Class II – symptomatic during ordinary activity
2) Willingness to participate in the study with signed informed consent.
7.2.2 Patient Exclusion
1.
Patients with evidence of renal parenchymal disease.
(Elevated serum BUN and creatinine; presence of urinary casts).
2.
History and evidence of liver disease, bronchial asthma,
Moderate to severe hypertension.
3. Pregnant and lactating women
4.
History of diuretic use within 24 hours and corticosteroid
therapy intake within 3 months of entry into the clinical trial.
7.2.3 General Objective
1. To determine the safety and efficacy of sambong tablets as diuretic in patients with altered fluid and electrolyte states manifested as edema.
7.2.4 Specific Objective
1. To determine the dose of sambong that will reduce edema and induce sodium and chloride excretion and increased urinary volume.
2. To compare the effects of sambong and furosemide in increasing urinary volume and electrolytes excretion among patients with edema.
3. To determine the adverse effects of sambong in comparison with furosemide after 24- hour dosing.
7.2.5 Methodology
All patients were admitted at the PGH Clinical Research Unit after satisfying the inclusion criteria and laboratory screening. On admission patients were informed of the nature of the study and were asked to sign consent forms. Baseline laboratory examinations were undertaken (CBC, FBS, BUN, Creatinine, SGOT, SGPT, Total protein, Albumin, Globulin, urinalysis and ECG). The patients were then placed on limited sodium diet of 50 mEq/day. Fluid intake was not controlled but volume of fluid intake and outputs were monitored.
Prior to administration of either sambong or thiazide on Day 2, 24-hour urine collection was started for volume and electrolytes (sodium potassium and chloride) determination. On Day 3, fasting serum electrolytes were done, after which the patients were given sambong tablets (TID) or hydrochlorthiazide (BID) tablets. Twenty-four hour urine collection for total volume and electrolyte determination was again done. Patients were closely observed for any possible adverse effects. Blood pressure, respiratory rate and heart rate were monitored. Serum electrolytes were determined 12 hours after the last dose of either sambong or thiazides.
On Day 4, patients given Sambong and those still with the need for diuretics were given standard diuretic (furosemide).
The dosages of sambong administered ranged from 32-50 mg/kg BW in 3 divided doses (tid) and hydrochlorthiazide was given at 50 mg twice a day (bid).
7.2.6 Result:
7.2.6.1 Patient Demographic Characteristics
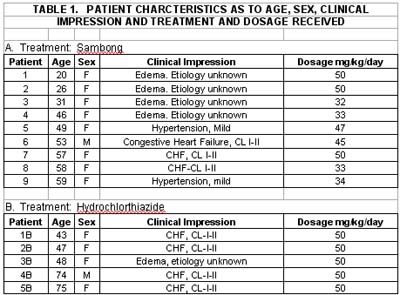
Patients administered sambong tablets numbered 9 while 5 were given hydrochlorthiazide. The age range in the sambong group was 20-59 years while those in the thiazide was 43-75 with mean age of 44.3 years for the sambong and 57.4 years for the thiazide group. Majority of the patients were females with only one male in each of the group. As to the cause of edema in the sambong group, 3 were with the congestive heart failure (Class I-II), 2 with mild hypertension and 4 with edema of unknown etiology. Among group II patients, 4 were with CHF, Class I-II and one with edema of unknown etiology. (Table I).
Comparison of baseline laboratory results showed mean values within normal limits for all parameters used. However, one patient in the sambong group had elevated SGPT while in the thiazide group one patient had slightly elevated SGOT and another patient with slightly elevated SGOT level. Urinalysis results were within normal limits for the two groups. ECG in six (6) of the sambong groups were within normal limits, 2 showed non-specific ST changes and one with borderline right atrial dilatation. In the thiazide group, ECG was normal in 1, 2 with non-specific ST changes and 2 showed left ventricular hypertrophy by voltage. (Table 2).
7.2.6.2 Urinary Output and Fluid Intake
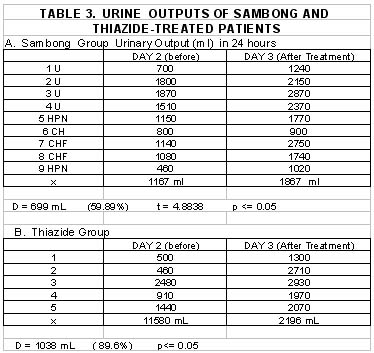
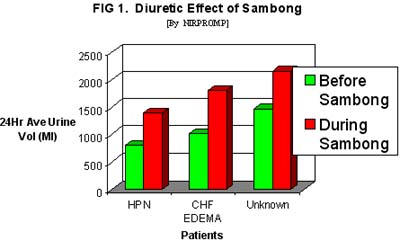
There was increased 24-hour-urinary output after either sambong or thiazide treatment. In the sambong group a mean value of 1167 mL urinary volume was obtained on Day 2 and 1867 mL by Day 3 resulting in and increase of 59.89% or 699 mL which was statistically significant P<= 0.05. Among the thiazide-treated the Day 2 Day 3 urinary volumes were 1158 mL and 2196 mL respectively, with a mean increase of 89.6% (1038 ml), which was also statistically significant. Analysis of the mean increase of urinary output effected by sambong compared with thiazide-induced did not show any significant statistical difference at p <=0.05. This implies that at the doses used, sambong can induce an increase in urinary volume comparable with that produced by thiazide (Table 3) ( Figure 1).
Evaluation of mean 24 hour fluid intake before and after treatment with sambong or thiazide and showed sambong effected an increased intake of 53.3 mL while post-thiazide fluid intake amounted to 692 mL, both of which were statistically significant (Table 4).
7.2.6.3 Urinary and Serum Electrolytes
Sambong treatment among patients with water and electrolyte retention produced increased excretion of sodium, potassium and chloride at 20.9%, 16.9% and 33.5% respectively. Statistical analysis showed that the only increase which was statistically significant was the chloriuresis at p<= 0.05. Thiazide administration produced urinary increase in the 3 electrolytes: 211% for sodium, 144% for potassium and 108% for chloride, which were all statistically significant. Comparison of the natriuresis and kaliuresis induced by sambong and thiazide showed significantly greater excretion of sodium and potassium by thiazides. However, the chloride excretion induced by sambong was not statistically different from that effected by thiazides and the chloriuresis by the two were therefore comparable.
The electrolyte serum level changes induced by either sambong or thiazide administration was not statistically significant when pre and post-dosing values were compared.
7.2.6.4 Onset of Effect
The time interval from sambong intake to time of first urine voiding was recorded for each patient. Sambong showed demonstrable diuretic effect from as early as 23 minutes to as long as 3 hours and 7 minutes (187 minutes) with a mean time interval of 65 minutes.
7.2.6.5 Adverse Effects
Monitoring for any untoward effects showed that a patient complained of only flatulence. Blood pressures and other vital signs remained within baseline normal limits during sambong or thiazide administration.
7.2.7 Discussion
Sambong therapy produced significant diuresis in 9 patients which was comparable with the diuresis induced by hydrochlorthiazide treatment.
Despite restriction of dietary sodium chloride intake at 50 mEq/ 24 hrs, sambong still produced a net increase in sodium excretion. Normally such sodium dietary restriction is associated with an immediate decrease in sodium urinary excretion so as to achieve sodium balance within 3 to 5 days. On statistical analysis however, the sambong-induced natriuresis was not statistically significant.
Potassium excretion was also increased by sambong but to a much less degree than that produced by thiazide. This is in contrast with the potassium-sparing effect produced by sambong among patients without altered electrolyte states. However, when the kaliuresis secondary to sambong dosing is compared with that due to thiazide which is statistically significant, sambong can be considered as having some potassium-sparing effects. Statistically significant chloride urinary excretion was observed with both sambong and thiazide administration and there was no statistically significant difference when these effects were compared. Serum electrolytes were not significantly altered by administration of either sambong or thiazide.
Concurrent with the diuresis and electrolyte excretion induced by sambong and thiazide, fluid intake when not restricted, was observed to be increased. This increase in fluid intake could be explained by the stimulation of the thirst mechanism caused by the decrease in intravascular fluid volume. The question arises on whether the diuresis is secondary to increased water intake alone. Water can induce diuresis when taken on a background of normal fluid and electrolyte state ( i.e. patients drinking large amounts of water without any previous dose). However, when an increased amount is taken in the presence of a negative fluid and electrolyte balance (i.e. patients with GIT / renal loses) the additional water will only fill up the contracted extracellular/ intravascular volume and will not produce the expected diuresis and instead will demonstrate increase in reabsorption of urinary electrolytes ( sodium and chloride) because of the contracted extracellular fluid volume. In this clinical trial, all patients exhibited increase urinary output as well as increased excretion of sodium and chloride. Thus, the increase in fluid intake in the patients was the result of renal loses of water and electrolytes.
Close examination of the varying doses and the increase in urinary output showed that there was no dose-effect relationship. At doses of 32-34 mg/kg the mean urinary increase was 727.5 mL; at 45-47 mg/kg BW the mean increase was 640 mL and at 50 mg/kg the mean volume, increase was 736.6 , with wide variability for each dosage range.
The mean time interval between sambong intake and onset of action ( time of first voiding) was 65 minutes. Thiazide diuretics have shown demonstrable diuretic effect one hour post-dosing.
Monitoring of vital signs showed that these were maintained at the baseline levels during sambong administration. However, in the two hypertensives, sambong effected a decrease in blood pressure to normal levels as was the case in the hypertensive patient given thiazide. Other vital signs were not significantly changed. Except in one sambong treated patient, who complained of flatulence, the rest of the patients did not complain of any untoward effects.
By definition, diuresis is an increase in urinary flow rate and any pharmacologic agent which is capable of promoting diuresis is called a diuretic. In general, diuretics are administered to achieve one of the two therapeutic goals: an increase in the urinary flow rate per minute or a negative balance of solute and water.
Based on the results from this clinical trial, sambong tablet at doses of 32 to 50 mg/kg BW given orally in three divided doses could be classified as a diuretic by producing the following:
A.
Therapeutic effects in patients with edema secondary to various etiologies
(CHF, mild Hypertension, etc) by producing statistically significant:
i. Increase in urinary volume.
ii. Increase in chloride excretion
(chloriuresis) and;
iii. Moderate increase in sodium excretion (natriuresis).
iv. Minimal increase in potassium excretion (possibly potassium sparing)
If these effects are compared with other diuretics, to what classification
does sambong belong? At present, diuretics are classified according to their
mechanism of action on different segments of the renal tubules. Group 1 diuretic,
with acetazolamide as prototype, primarily acts by inhibiting sodium reabsorption
on the proximal convoluted tubules. Group II diuretics, act principally on
the thick ascending loop of Henle by inhibiting active chloride reabsorption.
Furosemide and bumetanide belong to this class of the most potent diuretics.
The thiazide diuretics belong to the third group and produces diuretic effects
by its action on the cortical diluting segment of the nephron. All these three
classes can produce significant increase in sodium, potassium and chloride
excretion at varying degrees. The fourth class or group is the only one that
possesses potassium-sparing property (amiloride, triamterene, and spironolactones
are prototypes) by its action on the distal tubule and collecting duct. To
what classification does sambong belong? Since sambong produced significant
volume excretion and chloriuresis with some potassium-sparing effect, sambong
cannot be classified in any of the 4 classes of diuretics and may act along
more than one segment of the nephron.
Sambong is also not an osmotic diuretic like mannitol, which produces diuretic effect by being poorly reabsorbed by the renal tubules, and also hardly absorbed from the gastrointestinal tract. Sambong induces diuresis when given orally with an onset of action 65 minutes after administration.
A diuretic which has properties similar to sambong is the mercurial group which can induce increased urinary volume and combined chloriuresis and natriuresis while depressing potassium tubular secretion. The various untoward reactions produced by mercurials (ventricular fibrillation and mercurial poisoning) lead to its loss of value in diuretic therapy. Sambong however, did not produce any significant or harmful adverse effects.
7.2.8 Conclusion
1. Sambong tablet when administered orally at doses of 32-50 mg/kg BW, in three divided doses can induce diuresis in patients with altered fluid and electrolyte states.
2. Sambong produced statistically significant diuresis and chloriuresis comparable to hydrochlorthiazide given at 50 mg in 2 divided doses.
3. The natriuresis produced by sambong is modest and not comparable with natriuresis produced by thiazides. The potassium loss secondary to sambong is much less compared to the kaliuretic effect of thiazides. In patients without altered fluid and electrolyte states, potassium sparing was significant after sambong administration.
4. There were no significant adverse reactions complained of by patients given sambong at doses of 32-50 mg/kg BW. Vital signs and serum electrolytes were maintained at baseline levels. In two patients with hypertension, blood pressure was noted to be lowered.




 |
|
|

|
|
Copyright © 2002 Altermed Corporation. All rights reserved.