|

|
| 1 |
If a mixture of hydrogen and nitrogen is burnt in the air, what will be the major product formed? |
|
|
|
water |
|
|
|
ammonia |
|
|
|
nitrogen monoxide |
|
|
|
nitric acid |
|
|
|
|
|

|
| 2 |
Ammonia gas can be easily prepared in the Laboratory by heating an ammonium compound with an alkali. But we do not use NH4NO3 in this case. Why? |
|
|
|
This compound is expensive |
|
|
|
It is explosive on heating |
|
|
|
Very little ammonia will be given out |
|
|
|
Toxic fumes will be given out other than ammonia |
|
|
|
|
|

|
| 3 |
Ammonia is dried by |
|
|
|
dilute hydrochloric acid |
|
|
|
calcium oxide |
|
|
|
anhydrous calcium chloride |
|
|
|
concentrated sulphuric acid |
|
|
|
|
|

|
| 4 |
A sample of fertilizer was warmed with aqueous sodium hydroxide and a gas was given out which turned the moisted red litmus paper to blue. This shows the gas |
|
|
|
acidic and the fertilizer has an ammonium compound |
|
|
|
alkaline and the fertilizer has a potassium compound |
|
|
|
alkaline and the fertilizer has an ammonium compound |
|
|
|
acidic and the fertilizer has a potassium compound |
|
|
|
|
|

|
| 5 |
How can ammonia be produced?
1. heating ammonium carbonate
2. mixing equal mole of nitrogen and hydrogen gas
3. warming the concentrated ammonia solution |
|
|
|
1 and 2 |
|
|
|
1 and 3 |
|
|
|
2 and 3 |
|
|
|
all the options |
|
|
|
|
|

|
| 6 |
Nitrogenous fertilizers are often leached to the streams and rivers. This may lead to
1. increase in the level of nitrates in the drinking water
2. air pollution
3. growth of algae in the sea |
|
|
|
1 and 2 |
|
|
|
1 and 3 |
|
|
|
2 and 3 |
|
|
|
all the options |
|
|
|
|
|

|
| 7 |
1st statement : Nitrogen is essential to life.
2nd statement: All the living things contain proteins and all the proteins have nitrogen. |
|
|
|
Both statements are true but the 2nd one is not a correct explanation of the 1st one. |
|
|
|
Only one statement is true while the other is false. |
|
|
|
Both statements are true and the 2nd one is a correct explanation of the 1st one. |
|
|
|
Both statements are false. |
|
|
|
|
|

|
| 8 |
1st statement: Urea is a slow-acting fertilizer.
2nd statement: It takes a longer time for that urea to react with water to give ammonia. |
|
|
|
Both statements are true but the 2nd one is not a correct explanation of the 1st one. |
|
|
|
Only one statement is true while the other is false. |
|
|
|
Both statements are true and the 2nd one is a correct explanation of the 1st one. |
|
|
|
Both statements are false. |
|
|
|
|
|

|
| 9 |
1st statement : Barium sulphate may be used as a white paint additive.
2nd statement: Barium sulphate is a white compound which is insoluble in water. |
|
|
|
Both statements are true but the 2nd one is not a correct explanation of the 1st one. |
|
|
|
Only one statement is true while the other is false. |
|
|
|
Both statements are true and the 2nd one is a correct explanation of the 1st one. |
|
|
|
Both statements are false. |
|
|
|
|
|

|
| 10 |
Which of the following statements concerning the Haber Process is INCORRECT ? |
|
|
|
Iron is used as a catalyst in the process. |
|
|
|
The reaction is reversible. |
|
|
|
The reaction is exothermic. |
|
|
|
The process is performed at one atmospheric pressure. |
|
|
|
|
|

|
| 11 |
Which of the following elements are essential for plant growth ?
(1) potassium
(2) phosphorus
(3) silicon
(4) nitrogen |
|
|
|
(1), (2) and (3) only |
|
|
|
(1), (2) and (4) only |
|
|
|
(1), (3) and (4) only |
|
|
|
(2), (3) and (4) only |
|
|
|
|
|

|
| 12 |
Which of the following substances are used to produce nitric acid in industry ?
(1) air
(2) water
(3) oxygen
(4) ammonia |
|
|
|
(1), (2) and (3) only |
|
|
|
(1), (2) and (4) only |
|
|
|
(1), (3) and (4) only |
|
|
|
(2), (3) and (4) only |
|
|
|
|
|

|
| 13 |
Which of the following is a common use of ammonium nitrate ? |
|
|
|
to cure plant diseases |
|
|
|
to retard the growth of weeds |
|
|
|
to increase crop yield |
|
|
|
to neutralize acidic soil |
|
|
|
|
|

|
| 14 |
In the Haber process, the following reaction occurs :
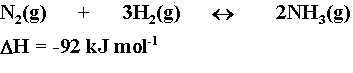
Which of the following statements concerning this reaction is correct ? |
|
|
|
The forward reaction is endothermic. |
|
|
|
The forward reaction can go to completion if sufficient amounts of nitrogen and hydrogen are used. |
|
|
|
In the catalytic conversion chamber, nitrogen, hydrogen and ammonia are all present. |
|
|
|
The optimum conditions for the Haber process are 10 atm and 500oC |
|
|
|
|
|

|
| 15 |
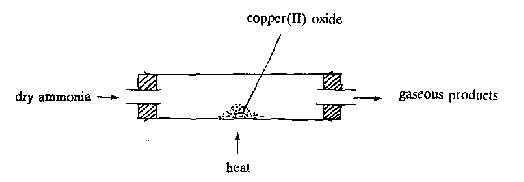
In the above experiment, the gaseous products are |
|
|
|
nitrogen and water vapour. |
|
|
|
nitrogen and hydrogen. |
|
|
|
nitrogen monoxide and water vapour. |
|
|
|
nitrogen monoxide and hydrogen. |
|
|
|
|
|

|