|

|
| 1 |
Nitric acid is not used |
|
|
|
in the making of explosive |
|
|
|
in producing the dyes |
|
|
|
in refining precious metals |
|
|
|
in making the soapless detergent |
|
|
|
|
|

|
| 2 |
Which of the following experiments is potentially hazardous and should NOT be carried out on laboratory bench ? |
|
|
|
heating ammonium nitrate strongly in a test tube |
|
|
|
passing ethene through bromine water in a conical flask |
|
|
|
adding small piece of sodium to a beaker of ethanol |
|
|
|
pouring a small amount of concentrated nitric acid into a large amount of water |
|
|
|
|
|

|
| 3 |
1st statement: A flash of lightning causes nitrogen monoxide to be formed in the air.
2nd statement: Lightning provides sufficient energy for nitrogen to react with oxygen in the air. |
|
|
|
Both statements are true but the 2nd one is not a correct explanation of the 1st one. |
|
|
|
Only one statement is true while the other is false. |
|
|
|
Both statements are true and the 2nd one is a correct explanation of the 1st one. |
|
|
|
Both statements are false. |
|
|
|
|
|

|
| 4 |
Which of the following oxidation numbers can nitrogen display in its compounds ?
(1) -3
(2) +2
(3) +3
(4) +4 |
|
|
|
(1), (2) and (4) only |
|
|
|
(2, (3) and (4) only |
|
|
|
(1), (2) and (3) only |
|
|
|
(1), (2), (3) and (4) |
|
|
|
|
|

|
| 5 |
Which of the following processes would produce nitrogen dioxide ?
(1) the action of heat on calcium nitrate
(2) the action of heat on ammonium nitrate
(3) the action of concentrated nitric acid on zinc |
|
|
|
(1) and (2) only |
|
|
|
(1) and (3) only |
|
|
|
(2) and (3) only |
|
|
|
(1), (2) and (3) |
|
|
|
|
|

|
| 6 |
Which of the following methods can be used to produce ammonia ?
(1) heating ammonium carbonate
(2) heating a mixture of ammonium sulphate and calcium oxide
(3) mixing 1 volume of nitrogen and 3 volumes of hydrogen at room temperature and pressure |
|
|
|
(1) and (2) only |
|
|
|
(1) and (3) only |
|
|
|
(2) and (3) only |
|
|
|
(1), (2) and (3) |
|
|
|
|
|

|
| 7 |
Which of the following nitrates would give off brown fumes when heated ?
(1) NaNO3
(2) AgNO3
(3) NH4NO3
(4) Pb(NO3)2 |
|
|
|
(1) and (3) only |
|
|
|
(2) and (4) only |
|
|
|
(1), (2) and (4) only |
|
|
|
(1), (3) and (4) only |
|
|
|
|
|

|
| 8 |
Assuming that the effectiveness of a nitrogen-containing fertilizer is proportional to its nitrogen content, which of the following compounds would be most effective as a fertilizer ?
fertilizer Relative molecular mass |
|
|
|
CO(NH2)2 60 |
|
|
|
NH4NO3 80 |
|
|
|
KNO3 101 |
|
|
|
(NH4)2SO4 132 |
|
|
|
|
|

|
| 9 |
1st statement : Nitrogen does not react readily with other elements or compounds.
2nd statement: The outermost electron shell of the nitrogen atom is completely filled. |
|
|
|
Both statements are true but the 2nd one is not a correct explanation of the 1st one. |
|
|
|
Only one statement is true while the other is false. |
|
|
|
Both statements are true and the 2nd one is a correct explanation of the 1st one. |
|
|
|
Both statements are false. |
|
|
|
|
|

|
| 10 |
1st statement : In ammonia, the mass of nitrogen is three times that of hydrogen.
2nd statement: For every nitrogen atom present in the ammonia molecule there are three hydrogen atoms. |
|
|
|
Both statements are true but the 2nd one is not a correct explanation of the 1st one. |
|
|
|
Only one statement is true while the other is false. |
|
|
|
Both statements are true and the 2nd one is a correct explanation of the 1st one. |
|
|
|
Both statements are false. |
|
|
|
|
|

|
| 11 |
1st statement : All nitrates are decomposed by heat.
2nd statement: Nitrates are oxidizing agents. |
|
|
|
Both statements are true but the 2nd one is not a correct explanation of the 1st one. |
|
|
|
Only one statement is true while the other is false. |
|
|
|
Both statements are true and the 2nd one is a correct explanation of the 1st one. |
|
|
|
Both statements are false. |
|
|
|
|
|

|
| 12 |
Oxygen is bubbled slowly into a concentrated ammonia solution as shown in the diagram below. 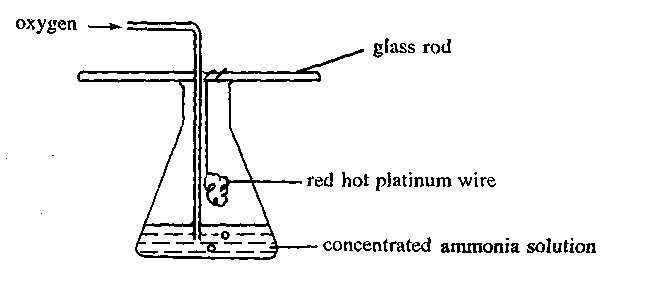
Which of the following statements concerning the experiment are correct ?
(1) A brown gas is formed in the flask.
(2) The platinum wire remains red hot throughout the experiment.
(3) A chemical reaction occurs at the surface of the platinum wire. |
|
|
|
(1) and (2) only |
|
|
|
(1) and (3) only |
|
|
|
(2) and (3) only |
|
|
|
(1), (2) and (3) |
|
|
|
|
|

|