|

|
| 1 |
In the Contact Process, the sulphur trioxide produced is converted to sulphuric acid by |
|
|
|
dissolving directly in water. |
|
|
|
dissolving in dilute sulphuric acid. |
|
|
|
dissolving in concentrated sulphuric and then diluting with water. |
|
|
|
dissolving in oleum and then diluting with water. |
|
|
|
|
|

|
| 2 |
A piece of filter paper soaked in warm turpentine is dropped into a gas jar of chlorine. Which of the following are observed ?
1. the turpentine burns spontaneously.
2. Black specks are produced.
3. White fumes are formed. |
|
|
|
(1) and (2) only |
|
|
|
(1) and (3) only |
|
|
|
(2) and (3) only |
|
|
|
(1), (2) and (3) |
|
|
|
|
|

|
| 3 |
Solutions of sodium chloride, sodium bromide and sodium iodide may be distinguished from each other by treating them separately with
1. chlorine water.
2. bromine water.
3. tetrachloromethane.
4. silver nitrate solution and aqueous ammonia. |
|
|
|
(1) and (3) only |
|
|
|
(1) and (4) only |
|
|
|
(2) and (3) only |
|
|
|
(2) and (4) only |
|
|
|
|
|

|
| 4 |
When chlorine is bubbled into an aqueous solution of compound X, the solution first turns brown, and then some dark coloured crystals are formed. X could be |
|
|
|
silver nitrate. |
|
|
|
potassium iodide. |
|
|
|
potassium sulphate. |
|
|
|
sodium bromide. |
|
|
|
|
|

|
| 5 |
When dry chlorine is passed over red-hot iron filings, which of the following would occur ? |
|
|
|
Iron(III) chloride is formed. |
|
|
|
Iron(II) chloride is formed. |
|
|
|
A mixture of iron(II) chloride and iron(III) chloride is formed. |
|
|
|
There is no reaction. |
|
|
|
|
|

|
| 6 |
Which of the following statements is/are true for chlorine, bromine and iodine ?
1. They exist as diatomic molecules at room temperature and pressure.
2. Their oxidizing power decreases as their relative molecular masses.
3. Their colours darken as their relative molecular masses increase. |
|
|
|
(1) only |
|
|
|
(1) and (3) only |
|
|
|
(2) and (3) only |
|
|
|
(1), (2) and (3) |
|
|
|
|
|

|
| 7 |
Chlorine gas |
|
|
|
decolorized bromine water. |
|
|
|
liberates iodine from potassium iodide solution. |
|
|
|
turns acidified potassium dichromate solution form orange to green. |
|
|
|
turns iron(III) chloride solution from yellow to green. |
|
|
|
|
|

|
| 8 |
Some chlorine water was exposed to sunlight (Diagram A);
a gas X was collected after some time (Diagram B).

Which of the following statements concerning gas X is correct ? |
|
|
|
It can relight a glowing splint. |
|
|
|
It has a pungent smell. |
|
|
|
It can turn wet blue litmus paper red and then white. |
|
|
|
It can give a 'POP' sound with a burning splint. |
|
|
|
|
|

|
| 9 |
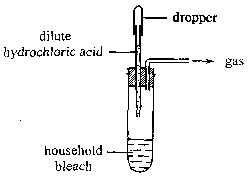
Which of the following statements concerning the gas produced in the above experiment are correct ?
(1) It is toxic.
(2) It is readily soluble in 1,1,1-trichloroethane.
(3) It can turn red litmus solution blue and then colourless. |
|
|
|
(1) and (2) only |
|
|
|
(1) and (3) only |
|
|
|
(2) and (3) only |
|
|
|
(1), (2) and (3) |
|
|
|
|
|

|