In the March 2001 issue of this journal Role of Homocysteine in causation of atherosclerosis was discussed. Several studies [Boushey et al JAMA1995; 274:1049-57, Nygard O et al NEJM 1997; 337:230-6, ECAP study, Chambers JC et al Lancet 2000; 355(9203):523-7, Study on South Asians (SHARE) by Anand S.S. et al Lancet 2000; 356:279-84 and Cochin Study] have clearly shown the correlation between homocysteine levels and atherosclerosis related disorders. It is quite clear now that homocysteine is as important a risk factor as cholesterol in causation of atherosclerosis, and more importantly so in people of the Indian subcontinent. In this issue is discussed briefly the management of hyperhomocysteinemia.
As variable changes in homocyst(e)ine levels have been observed postprandially, it is customary to obtain measurements in the fasting state. The majority of the clinical studies involving homocysteine have relied on the measurement of homocyst(e)ine. Levels of homocyst(e)ine have been remarkably similar between laboratories in studies conducted by different investigators using several methods. In India, importance of estimating plasma homocyst(e)ine levels to evaluate the risk of Atherosclerotic Vascular Disease is increasingly getting recognition. Apart from a few government research institutes, at least 2 cities, Mumbai and Delhi, have facilities for estimating plasma homocyst(e)ine. There are 2 estimation procedures which can be used. The commonly used one is based on the enzyme- linked immunosorbent assay (ELISA) technique, and costs -Rs. 800-1,000. The other, using high pressure liquid chromatography (HPLC), is much more expensive. As such, there are no special precautions or requirements for the patients; a fasting blood sample is collected.
An oral dose of methionine (100 mg/kg of body weight) can be given to persons with suspected hyperhomocyst(e)inemia who have normal fasting homocyst(e)ine concentrations. The homocyst(e)ine levels are determined before the methionine challenge and between four and eight hours afterward. Hyperhomocyst(e)inemia is considered to be present if the homocyst(e)ine concentration after methionine challenge is more than 2 standard deviation (SD) above the mean. Although multiple sampling strategies have been described, the 2-hour test has been validated extensively and it seems more practical than later blood sampling. This test may uncover 39% of subjects with homocyst(e)ine related cardiovascular disease risk but with normal basal homocyst(e)ine levels.
The prognostic value of the methionine-challenge test has recently been criticized. In persons with the thermolabile variant of MTHFR, there was only a weak association between homocyst(e)ine concentrations after methionine challenge and premature CHD (odds ratio 1.7; p = 0.12), whereas there was a significant association between fasting homocyst(e)ine concentrations and premature CHD (odds ratio 2.0; p = 0.04). So in most studies fasting Homocysteine level has been used.
The treatment of hyperhomocyst(e)inemia varies with the underlying cause; however, vitamin supplementation with folic acid, pyridoxine, and vitamin B12 is generally effective in reducing homocyst(e)ine concentrations. Homocyst(e)ine levels are inversely related to blood concentrations of folate, vitamin B12 and, to a lesser extent, vitamin B6. Dietary supplements of these vitamins are used to reduce homocysteine concentrations in subjects with homozygous homocystinuria, who have particularly high blood concentrations homocysteine. Several randomized controlled trials of the effects of folic acid supplements on homocysteine concentrations have been conducted over the last decade. In most patients, small doses of folate rapidly decrease homocyst(e)ine concentrations. Folic acid alone, folic acid combined with vitamins B12 and B6, and vitamins B6 and B12 have been shown to reduce homocyst(e)ine concentrations. Normalization of homocyst(e)ine levels usually occurs within 4-6 weeks after the initiation of therapy, but may occur in as little as two weeks. The minimal effective doses of folic acid, and vitamins B6 and B12 have yet to be determined. Administration of supplemental folic acid in doses between 0.2 and 15 mg/d can lower plasma homocyst(e)ine levels without apparent toxicity. In a placebo-controlled study, combination of multiple agents including folic acid (0.65 mg/d), vitamin B6 (10 mg/d), a vitamin B12 (0.4 mg/d) was very effective in reducing homocyst(e)ine levels in patients with moderate or intermediate hyper-homocyst(e)inemia. Let us examine the available evidence try and determine the dose of folic acid which will adequately reduce and maintain homocyst(e)ine within normal levels. A good beginning would be to examine the results of the Homocysteine Lowering Trialists Collaboration (HLTC) Study.
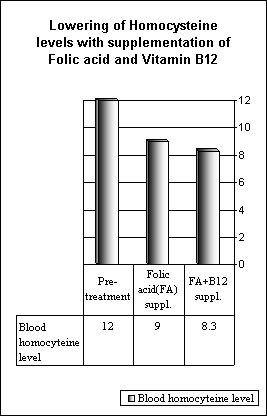
The HLTC was ameta-analysis of 12 randomized, controlled trials, involving 1,114 patients , that assessed the effects of folic acid based supplements on homocyst(e)ine. This study aimed to determine the size of the reduction in homocyst(e)ine achieved with different doses of folic acid, and with the addition of vitamin B12 and vitamin B6. Multivariate regression analysis was used to determine the effects on homocysteine concentrations of different doses of folic acid and of the addition of vitamin B12 or B6. Among the 1,114 subjects in the trials, the mean age was 52 years (range of trial means: 23-75 years) and the mean duration of treatment was 6 weeks (range 3-12 weeks). The median pretreatment blood concentration of homocysteine was 11.8I.micromol/L and of folate was 11.6 nmol/L. Effect of folic acid supplementation After pretreatment homocyst(e)ine and folate concentrations were adjusted for, there was no longer much evidence of heterogeneity between the separate homocyst(e)ine lowering effects in the different trials of daily folic acid doses of < 1 mg (mean dose 0.5 mg; p value for heterogeneity = 0.15), of 1-3 mg (mean dose 1.2 mg; p = 0.05), or of > 3 mg folic acid (mean dose 5.7 mg; p = 0.69). Nor was there any evidence of differences between the homocyst(e)ine lowering effects of these different folic acid doses. For individuals with pretreatment homocyst(e)ine of 12Micromol/L and of folate of 12 nmol/L (approximate average concentrations for Western populations) , folic acid doses of < 1 mg, 1-3 mg, and > 3 mg daily were each associated with a 25% reduction in homocyst(e)ine . Effect of adding vitamin B12 or vitamin B6 to folic acid The addition of vitamin B12 (0.02-1 mg daily; mean 0.5 mg) to folic acid further reduced blood homocysteine concentrations by about 7% (3% to 10%).Hence, among people with pretreatment blood concentrations of homocysteine of 12 Micromol/L and of folate of 12 nmol/L, adding vitamin B12 to folic acid increased the reduction in homocysteine from 25% (23% to 28%) to 31% (27% to 35%). Adding vitamin B6 (2-50 mg daily; mean 16.5 mg) to folic acid did not lower blood homocysteine any further.
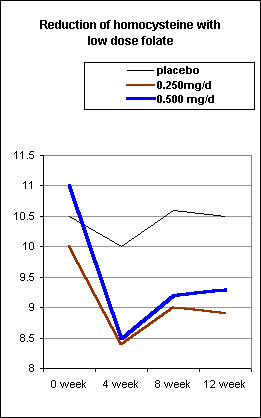
Recent evidence suggests that even small physiological doses of folic acid are adequate to reduce blood homocyst(e)ine. Brouwer et al. studied the effect of low-dose folic acid administration (250 or 500 microgram/day) for 4 weeks, on homocyst(e)ine and folate status. In this placebo-controlled study, 144 healthy women aged 18-40 years received 500 microgram folic acid/day, 500 microgram folic acid every second day (average 250 microgram/day) or a placebo along with their habitual diet (mean dietary folate intake: 280 microg/day). Administration of 250 and 500 microgram folic acid for 4 weeks significantly decreased homocyst(e)ine (p < 0.001) from the baseline, in both the 250 and 500 microgram groups. Further, 8 weeks after the end of the intervention period (week 12), homocyst(e)ine in the folic acid supplemented group had not returned to baseline (week 0). This study indicates that even small doses of 250 microgram/day of folic acid, in addition to that ingested from diet, were adequate to reduce and maintain homocyst(e)ine levels.
As the awareness of the problem of hyperhomocyst(e)inemia increases the testing of homocysteine blood levels will become more widely available and cheaper. Hyperhomocysteinemia has particular relevance to people of south asian origin as the levels of homocysteine are higher and the levels of folate are lower in them. Moreover south Asians are more susceptible to Atherosclerosis due to syndrome X and some other factors. Homocysteine is as important a risk factor as cholesterol or smoking. Treatment of hyperhomocysteinemia is quite simply vitamin supplementation but it has to be continued life long to achieve any positive results.