The elimination of bullae and/or sectors of lung destroyed by emphysema (lung remodeling) is of demonstrated
efficacy in improving tolerance to exercise in certain dyspneic patients suffering from pure or predominantly panacinar emphysema.
However, the procedure has not met with universal acceptance, due to the complications created by air leakage
at the sutures of the emphysematous lung.
The original suturing technique described in the present study has been performed in 76 patients over a period of 11 years, and has
been found to be efficient in countering air leakage. The technique also involves a number of
maneuvers with the aim of preventing damage to the fragile lung in areas removed for the suture zone - this being a common cause of uncontrollable air
leakage.
The areas of destructive emphysema and the bullae produced in the lung with pure or predominantly panacinar emphysema (PAE)(1-10)
accentuate the deterioration of the organ's elastic rebound capacity. This again in turn leads to respiratory problems that adversely
affect tolerance to exercise and may cause discapacitating dyspnea (4-13). Likewise, the decreased inspiratory negative intrapleural pressure prevents
blood from adequately reaching the right atrium, thus causing a drop in cardiac output. This effect is eventually reflected
by muscle atrophy and diminished cutaneous perfusion in these patients.
The adoption of surgical measures to eliminate the above mentioned lesions may improve the situation (2-4,6,8,14). Frequently, however, the mere surgical removal of the bullae
is insufficient to improve the condition, and complementary measures may be required in the form
of the elimination of coexisting destructive emphysematous foci (4-6). In those cases were few bullae are observed, surgery
centers on the elimination of severe or destructive emphysematous areas that ultimately exert effects similar to those of bulae
(2-4,6,14). For this reason, we prefer to abandon the concept of bullectomy in favor of "lung remodeling". Two phenomena occur when emphysematous destruction affects a particular
pulmonary zone:
(b) the retracted tissue surrounding the distended zone in turn loses rebound capacity, for on retracting and shortening,
its elastic fibers no longer exert a tension effect. Consequently, elastic rebounding capacity decreases in PAE as a result of two mechanisms. One, destruction
of the elastic meshwork, is irreversible, while the resting condition of the retracted sector fibers is reversible.
The aim of lung remodeling is to eliminate those parenchymal regions that have lost their rebound capacity due to destruction, thus
restoring tension to the inactive elastic tissue of the retracted territories.
The removal of bullae tends to involve fewer problems than the elimination of emphysematous lung tissue, due to the air leakage
generated by suturing in the latter case (2,3,15). The preoperative differentiation (clinical examination, radiology, functional tests) between a bulla and a sector of lung
destroyed by PAE is difficult to establish. In this sense, we consider that the surgeon should be able to operate both procedures
with the same margin of safety (12). This is the aim of the present technique.
MATERIAL AND METHODS
The application of this technique is based on the fact that the PAE lesion predominate in the
peripheral regions of the lung, leaving the central areas more intact (3,10); a resistant plane is established on which to apply the sutures, formed by the tissues to be eliminate.
To this effect we employ two identical instruments (Fig. 1).
This surgery is based on the fact that the panacinar emphysematous lesions exhibit an irregular distribution (3,4,8,14), in which the same lung may present
emphysematous destruction coexisting with moderate or even slight lesions. The severity of each particular case depends upon
the extent and distribution of the different degrees of lesion.(a) the damaged region is able to distend due to retraction on the part of the surrounding tissue, which preserves its elastic
elements; and
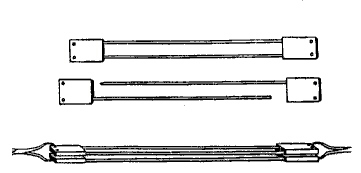
Each in turn consists of two similar pieces that are assembled to prepare the tissue for suturing, and then deassembled on copleting the procedure. Each piece presents a flat rectangular head unit out of which a thin metal rod some 12 cm long emerges. At the other end of the same face of the head unit there is a deep, dead-end orifice into which the rod of the opposing head unit is introduced. Each head unit in turn possesses two transverse orifices for manipulating the units during the assembly process. One of the rods ends in a point, while the tip of the other is rounded and is a few millimeters shorter, to facilitate coupling.
The lobe to be remodeled is exteriorized and insuflated. The most damaged section is easily distended.A small incision is made at the most distal point (Fig. 2).

The cortical zone with destructive emphysema rapidly collapses, clearly delimiting the line bordering with less damaged tissue. At this point we temporarily apply a straight clamp to block air escape (Fig. 3).

The blocked zone is divided into two flaps down to the clamp; care is taken to not perforate them. The needle point rod is then inserted along the full length of the free margin of one of the flaps (Fig. 4), followed by assembly with the complementing head unit and blunt rod.
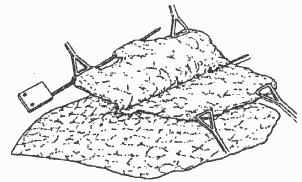
Following assembly, the flap is rolled up (Fig. 5).

The resulting flap roll lies parallel to the blocking clamp and is fixed by two forceps through one of the lateral orifices of each head unid (Fig. 5). The same procedure is repeated with the other parenchymnal flap. The clamp and forceps are removed, and the instruments are given a final twist to superficialize the deepest zone of the incision. The head units of both instruments are precisely opposed and fixed in position by two forceps through the deepest transverse orifices of the head units (Fig. 6).

Thus, by using the fragile emphysematous lung tissue, a firm plane is created on which to support suturing. At each of the incision comissures (critical suture points) we apply two silk x-sutures (Fig. 6). A horizontal "shoemaker's"surget is performed (thus termed in being similar to that used in sewing soles to shoes)(Fig. 7); multifilament 2-0 polyglactine lubricatet with silicone is employed to this effect.

Suturing begins at a point passing below the comissure. The free end of the filament (that without the needle) is deliberately left long and parallel to the suture trajectory. the needle advances as shown in Figure 7, and includes the free end at each turn. The needle end of the filament should be kept tense, so that at each turn it drags the free end of the filament into the tissue. The needle should pass through the planes perpendicularly, advancing only on the side opposite to the free end of the filament, to secure full tissue inclusion in the surget.
Having completed the trajectory, and always keeping the needle end of the filament firm, traction is exerted upon the free end so that the tissue becomes firmly trapped between the two threads. Both are then knotted, including the comissure. The instruments are deassembled and removed. The knot is tightened and reinforced by several more, superimposed upon each other.
This procedure thus affords an effective hemo- and pneumostatic suture. The few leakages left by the passage of the filament through the visceral pleura are covered by an extensive tissue flap that simultaneously facilitates cicatrization (Fig. 8).

The procedure is repeated as often as required until the lung presents a volume appropriate to the
thoracic capacity. This aspect is of fundamental importance. An excessively voluminous lung will be unable to secure an optimal
functional recovery of the recycled tissue, while too small a lung will be unable to expand sufficiently. This inconvenience is avoided by
correctly evaluating the collapsed sector via selective pulmonary cineangiography.
The cases of prolonged air leakage encountered in our series of 76 patients were not caused by the suturing but by lung tearings or lesions far removed from the operated site. In order to avoid such complication, a number of measures should be adopted:
- .The thoracotomy should be sufficiently extensive to facilitate maneuvering and avoid traction.
.A blunt instrument should be utilized to access to the pleural cavity.
.In sectioning the visceral pleura, care should be taken to not depress the lung with instruments, but protect it with the flexed index finger.
.Care must be taken to carefully debride the lung prior to exteriorization, to avoid the risk of tearing the organ. Such a tear would cause air leakage of difficult management. The emphysematous lesions are a constant presence at source bronchial level. In order to avoid possible tearing of the latter on exerting traction to expose the lung, the mediastinal pleura around the bronchus should be sectioned (Fig. 9).

.Care should be exerted to avoid rib fracture with the separators; the ribs of these patients tend to be fragile.
.The lung should be handled in a semi-insuflated state.
.Both the lung and hands of the surgeon should be kept permanently humid. Exposure to the air and the heat of the overhead illumination tnd to dry the visceral pleura of the emphysematous lung, which becomes even more fragile to contact with dry gloves.
. Metal instruments should be avoided in rib joining; the pericostal x-sutures effectively compensate them.
.Two-way sondes should be employed in anesthesia, to avoid excessive distension of the destructive emphysema zones of the contralateral lung during insuflation maneuvers to detect lung leakage.
- REFERENCES
1.CIBA Guest Symposium Report (1959). Terminology, definition and classification of chronic emphysema and relatet contitions. Thoraz, 14;286.
2. Current status of the surgical treatment of pulmonary emphysema and asthma. Am Rev Resp Dis, 97: 486,1969.
Delarue NC, Woolf CR, Sanders DE et al. (1977). Surgical treatment for pulmonary emphysema. Can J Surg.
4. Fitzgerald MX, Keeland PJ, Cugell DW (1974). Long-term results of surgery for bullous emphysema. J Thor and Cardiovasc Surg, 68(4).
5. Fletcher CM, Elmes PC, Frairbairn AS (1959). The significance of respiratory symptoms and the diagnosis of chronic bronchitis in the working population. Br. Med J. <6>6. Gaenssler EA, Cugel DW, Knudson RJ (1983). Surgical management of emphysema. Clinics in Chest Medicine, 4(3).
7. Jensen KM, Miscall L, Steinberg I (1961). Angiocardiography in bullous emphysema: Its role in selection of the case suitable for surgery. Am J Roentgenol, 85: 229.
8. Kress MB, Goco RV, Brantigan O (1968). The role of surgery in the management of generalized emphysema without blebls and bullae. Dis Chest, 53(4).
9. Parmar JM, Hubbard WG, Matthews HR (1987). Teflon strip pneumostasis for excision of giant emphysematous bullae. Thorax, 42(2).
10. Reid L (1966). Emphysema: Classification and clinical significance. J Dis Chest, 60.
11. Serrano-Munoz F, Alix-Trueba A, Toledo J et al.(1966). Tratamiento quirúrgico del enfisema pulmonar. Revista Clínica Española.
12. Seineldin S, Luque A, Talarn C et al. (1989). Reseccion de ampollas de enfisema por la técnica de Crosa-Dorado. Revista Argentina de Cirurgía, 56(5).
13. Sharp JT (1983). The respiratory muscles in emphysema. Clinics in Chest Medicine, 4(3).
14. Brantigan O, Cress M, Mueller EA (1961). The surgical approach to pulmonary emphysema. Am Rev Resp Dis, 80.
15. Bousy SF, Kohen R, Billig DM et al. (1968). Bullous emphysema: Clinical, roentgenological and physiological study of 49 patients. Dis Chest, 54(4).
 Literature Survey
Literature Survey

