
ANTIMICROBIAL
FINISHES

PRACHUR BHARGAVA
INDERPREET SINGH
|
S.no |
TOPIC |
Page no |
|
|
|
|
|
1 |
Introduction |
3 |
|
|
|
|
|
2 |
Microorganisms
and their effect on textiles. |
4 |
|
|
|
|
|
3 |
Anti-microbial-Agents |
7 |
|
|
|
|
|
4 |
Classes
of anti Microbial Agents |
9 |
|
|
|
|
|
5 |
Methods
of application of Anti-microbial finish. |
13 |
|
|
|
|
|
6 |
Effect
of Anti-microbial finishes on chemical structure. |
14 |
|
|
|
|
|
7 |
Effect
of Anti-microbial finishes on Mechanical Structure |
17 |
|
|
|
|
|
8 |
Assessment
of Anti-microbial Finishes |
19 |
|
|
|
|
|
9 |
Anti-odor
finishes |
22 |
|
|
|
|
|
10 |
Surgical
applications of anti-microbial finishes |
24 |
|
|
|
|
|
11 |
References |
25 |
Clothing and textile materials are carriers of
microorganisms such as pathogenic bacteria, odor-generating bacteria , and mould
fungi because of the adhesion of these microorganisms on fabric surfaces. Most
textile materials currently used in hospitals and hotels are conductive to
cross-infection or transmission of diseases caused by microorganisms. This has
created increased pressure for protection of personnel with functional clothing
and materials i.e. application of Anti-microbial Finishes.
In
this review first we have discussed about various microorganisms and their
effect on textile materials. Next we have discussed various Anti-microbial
finishes, their methods of application and their effects on the chemical and
mechanical properties of fabrics. One of the most important applications of
Anti-microbial Finishes is in Surgery and related health fields. In the last
section we have tried to highlight this important application of Antimicrobial
Finishes.
MICROORGANISMS
AND THEIR EFFECT ON TEXTILES
Microorganisms
are a part of our daily lives. They encounter and accompany the human being in
most different shapes –mostly unnoticed. Microorganisms i.e. bacteria, moulds
yeast are playing an important role in numerous biological reactions. The skin
is also crowded with innumerable organisms, which mostly are part of natural
skin pH value.
Smallest organisms
–biggest effects
Microorganisms are extremely adaptable. If the environment is
favorable certain bacteria grow in no time from single germ to millions Germs
very rapidly develop via cell division: every 20 minutes they double their
population
For their growth they mainly require humidity and as a culture
medium organic material is required which they attack and digest with enzymes.
This is how soil bacteria and moulds are doing their invaluable job as humus
producers. The same way however they destroy material of wealth for mankind.
Especially good environments for the microorganisms living on the skin are areas
with high humidity.
Microorganisms on textiles
Due to the close contact between skin and textiles after only a
short wearing times microorganisms crowd them. Additionally their environmental
conditions on textiles are similarly favorable as on the skin and thus support
the microbial growth.
Not all
microorganisms are alike.
A rough subdivision can be made as:
-Bacteria
-Yeast Fungi
-Virus
Bacteria: Bacteria
are categorized as
Gram
positive
Gram
negative
This classification goes back to the Danish pathologist Gram
(1884) who found the test method for diversification of cellulose membranes of
bacteria:
On the skin mostly Gram-positive
bacteria like Staphylococci and different Coryne bacteria are found.
Gram-negative
bacteria
like Escheria coli are found in feces.
Microorganisms are invisible but there presence can be easily
felt, smelt or even seen when they are uninhibited reproducing and then deploy
their activity. They produce different degradation products which man senses as
unpleasant smell growing to stench.
Bacteria e.g. convert sweat into stinking substances like carbon
acid aldehyde and amines.
The proliferation of the microorganisms causes destruction of the
material on which they are growing.
Fresh and
Hygienic?
It is wide spread belief that washed textiles are hygienic, fresh
and clean .A study shows that this belief is partially realistic. The following
test prove that this postulate:
50
test persons have been wearing for 25 days sanitized antibacterial finished wool
stockings and parallel stockings without treatment were tested. Everyday the
stockings have been washed at 40 C and have been worn again next day .In 5 days
intervals the quantity of bacteria before and after washing have been measured.
Independently of the number of washes the especially finished stockings showed
always the same fresh hygienic and clean results .The not so especially finished
stockings showed for a certain time after washing a reduced amount of bacteria.
However after that time the number of bacteria grew explosively.
Conditions
required for the growth of these organisms are:
Nutrients:
Soil,
dust and some textile finishes can all be sources of nutrients for
microorganism. Perspiration contains salts, amino acids, carboxylic acids and
other essential nutrients. Dead skin cells or oils secreted from the skin are
also a potential source of carbon. Cellulose itself is not a nutrient, but many
bacteria produce extra cellular cellulose enzymes, which convert cellulose into
the readily metabolized glucose.This has the dual effect of promoting further
growth, and degrading the Fiber.
Water
Porous fibers of hydrophilic nature provide
a suitable environment for
Growth of microorganism, Human beings have been estimated to have been
estimated to give off an average of 1OOg/ hour of water as perspiration, which
accumulates in clothing and bedding. A humid environment will provide enough
water to support fungal growth. Bacteria need more water and require the fabric
to be damp.
Oxygen
The atmosphere provides a ready source of
Oxygen.
Warmth
Most fungi and bacteria will grow at ambient temperatures of
10-200 C. Certain bacteria prefer the slightly warmer conditions of clothing or
bedding in close proximity to the skin. These bacteria such as Staphylococcus
aureus, S. epidennis and Corynebacterium sp. are those most commonly found on
the skin.
Affect of
Microorganism growth on fabric
In the majority of
cases, the presence and growth of microorganisms passes unnoticed in certain
circumstances however, undesirable and possibly serious effects can occur.
Health
The growth of certain microorganisms has been known to affect the
health of the user. The consequence of infection in some case may only be a sore
throat caused by Escherichia coli. S. aureus is known to infect skin, surgical
wounds and burns, and can have serious effects. Textile products such as
bedding, curtains, uniform and mops may be involved in the transport of bacteria
within hospitals. Athlete’s foot is
a common infection caused by the fungus Tricophyton mentagrophytes. The role of
socks in retaining moisture and nutrients may be significant in the growth of
this organism. Microorganisms may produce metabolites, which cause irritation or
allergic reactions. The bactedrium Proteus mirabilis and the yeast Candida
albicans are both capable of metabolizing urea to ammonia, which has been
proposed as a possible cause of diaper rash. The pH increase caused by the
ammonia may also irritate the skin.
Odors.
Bacterial metabolism of perspiration in moist, warm area such as
inside shoes, underarm of clothing and towels, produces the volatile molecules
such as carboxylic acids and aldehydes associated with body odor. Organisms
isolated from perspiration are those commonly found on the skin, such as S.
aureus, S. epidennidis, Corynebacterium sp. and Propionibacteria.
Fabric Deterioration
The cellulose enzymes produced by some bacteria and fungi are
capable of degrading cotton fibers, causing loss of strength, and reducing the
lifetime of the textile. Structural damage to fibers by S. aureus has been
reported Fungi may also produce unsightly staining, when pigments produced
diffuse into the fiber. In may case, these pigments cannot be removed by washing
or bleaching.
Antimicrobial
agents are chemical compositions that are used to prevent microbiological
contamination and deterioration of the commercial products, materials and
systems. Their areas of application include cosmetics, disinfectants, and
sanitizers, wood preservation, food and animal feed, paints, cooling water,
material working fluids, hospital and medical uses, plastics and resins,
petroleum, pulp and paper, textiles, latex, adhesives, leather and hides, and
paint slurries.
A given
antimicrobial agent may either destroy the entire microbe cell present
Or just prevent
their further proliferation to numbers that would be significantly destructive
to the substrate or system being protected. In sharp contrast to certain medical
situations that require complete sterility, most industrial applications demand
only that potentially destructive microorganisms are inhibited from
proliferating to a detrimental level.
Mode of Action
The mechanisms by which chemical agents exert antimicrobial activity
depend upon effective contact between the chemical and the microorganism and
involve disruptive interaction with a biochemical or physical component of the
organisms that is essential to its structure and metabolism. The targets may be
a single enzyme, a cell membrane, intercellular systems, cytoplasm, or
combinations of these and the nature
Of the action is dependent on the organisms and the
environment in which the interaction occurs as well as on the antimicrobial
agents
Some of the primary modes of
action are as follows:
Cell wall synthesis inhibitors:
Cell
wall synthesis inhibitors generally inhibit some step in the synthesis of
bacterial peptidoglycan
Cell membrane inhibitors:
They disorganize the structure or inhibit the function of
bacterial membranes. The integrity of the cytoplasmic and outer membranes is
vital to bacteria, and compounds that disorganize the membranes rapidly kill the
cells. However, due to the similarities in phospholipids in eubacterial and
eukaryotic membranes, this action is rarely specific enough to permit these
compounds to be used systemically.
Protein synthesis
inhibitors ~ Many therapeutically useful antibiotics owe their action to
inhibition of some step in the complex process of protein synthesis. Their
attack is always at one of the events occurring on the ribosome and never at the
stage of amino acid activation or attachment to a particular tRNA
Competitive Inhibitors:
The competitive inhibitors are mostly all synthetic
chemotherapeutic agents. Most are "growth factor analogs" which are
structurally similar to a bacterial growth factor but which do not fulfill its
metabolic function in the cell. Some are bacteriostatic and some are
bactericidal.
Effects on Nucleic Acids:
Some chemotherapeutic agents affect the synthesis of DNA or RNA,
or can bind to DNA or RNA so that their messages cannot be read. Either case, of
course, can block the growth of cells.
8
CLASSES OF ANTIMICROBIAL
AGENTS
Phenolics
Phenolics generally exhibit broad - spectrum activity against
Gram-positive and Cram-negative bacteria as well as against fungi. This property
plus their relatively moderate cost, make them quite cost-effective.
Phenols are considered to be moderately too highly toxic. The
chlorophenols can be absorbed through the skin in toxic levels and their dusts
are very irritating to the skin, eyes and respiratory tract. The formation of
highly toxic chlorinated dibenzop-dioxins
As
impurities in the production of various chlorophenols has been reported.
However, the acceptable products given below continue td keep the phenolic class
of antimicrobial agents among the most widely used in industry
|
NAME |
APPLICATION |
|
Pentacholoropenol |
Textile, wood and paper leather, paints |
|
Sodium pentachlorophenoxide |
Textiles, adhesives, leather, pulp and paper, water
treatment |
|
2,4,5-trichloropheno1 |
Textile, adhesives, rubber, paper mills, slim cides, leather |
|
Sodium o-phenyl-phenoxide |
Textiles, adhesives, ceramic, glazes, clay slips, paints,
floor waxes. |
Halogen compounds
The halogens constitute a class of antimicrobial agents that are
larger in commercial distribution than the phonetics. May of their applications,
e.g. swimming -pool sanitizers, household and hospital disinfectants, and
surgical scrubs are outside the scope of this article, but many industrial uses
exist, especially for a number of organic halogen derivatives.
The halogen can be
in the active or available form in which it demonstrates
antimicrobial
activity because of its oxidizing capacity through a positive valence
state. The halogen
also contributes activity as a covalent substituent of a complex structure. The
spectrum of commercial products ranges from the elements, to inorganic
hypohalites, to complex halogen -substituted organic compositions.
Iodine Compounds.
The
use of elemental iodine as antiseptic dates back to 1839 -. It is used today for
various medicinal purposes.
|
NAME |
APPLICATION |
|
p-tolydiiodomethyl
sulphone |
Textiles, paint
preservative, paint mildewcide, water based adhesives, |
OUATERNARY AMMONIUM
COMPOUNDS (OUATS)
The category
represents one of the largest, most diverse classes of antimicrobial
agents in use. Chemically, the products may be represented by the
general formula
|
|
The nitrogen atom carries four covalent bound Substituents that
give it a cationic charge. The R groups may be almost any organic substituents
that allow carbon nitrogen bonding with any permutation of like and unlike R
groups. The nitrogen atom may be part of a heterocyclic, aromatic structure,
thereby automatically fixing three substituents.
|
NAME |
APPLICATION |
|
3-(trimethoxysilyl)-propyldimethyloctadecylammonium
chloride |
Textiles |
Mercurils
|
NAME |
APPLICATION |
|
Di (phenyl
mercury)-dodecenylsuccinate (PDMS) |
Textiles, paint
preservative, paint mildewcide, |
Arsenicals:
These are used in diluted forms as could be toxic at high
concentrations. Their major
application is in plastic industry. 10,10 - Oxybisphenoxyarsine is
the only organ arsenic product of importance as an industrial antimicrobial
agent.
|
NAME |
APPLICATION |
|
10,10 -
oxybisphenoxy-arsine |
Textiles, plastics |
Organotins.
Copper Compounds.
|
NAME |
APPLICATION |
|
Copper
8-quinolinate |
Textiles,
plastics, wood |
|
Copper naphthenate |
Textiles, wood |
Anilides
Trichlorocarbanilide (TCC) is the only anilide that enjoy sizable
sales as an antimicrobial agent; it is used almost exclusively in deodorant bar
soaps. The FDA's Advisory Review Panel on over - the counter antimicrobial drug
products critically reviewed appropriate data regarding the safety of
salicylanilides and carbanilides, and found considerable evidence of
photosensitization by halogenated salicyolanilides. The toxicology has been
discussed in various articles. Subsequently the FDA placed halogenated
salicylanilides in Category2 as not safe and effective for use in drug product
and cosmetics.
|
NAME |
APPLICATION |
|
Salicylanilides |
Textiles, leather |
miscellaneous compounds.
A few antimicrobial agents are unique in their functional
classification. In general, each has one significant main use and, in most
cases, only limited market significance as an industrial antimicrobial agent.
The significant products are shown in the Table. Organic acids and their salts
constitute a sizable category among antimicrobial agents.
|
NAME |
APPLICATION |
|
2,6-dimethyl,
1,3-dioxanol-4 acetate |
Textiles |
CHOOSING
AN ANTI
MICROBIAL AGENT
·
Is claimed to combat the microorganism of
concern to the degree desired.
·
Appears
to be physically and chemically compatible with the system, i.e. will not
upset desirable physical and chemical properties of the system and
(conversely) will not be inactivated by the ingredients of the system.
·
Maintains
stability under use and storage conditions (pH, temperature, light, etc) for the
required length of time.
·
Is
safe and nontoxic in handling, formulation and use.
·
Is environmentally acceptable, and
·
Is
economically acceptable and cost-effective
Application
methods are particularly suitable:
1.Padding:
Fabric is run through a pad- bath to give approximately I% of treatment fabric
is then padded wet on -wet through a bath of approximately 0.5% Repulix, rinsed
and dried.
Other
finishes may also be applied simultaneously through slight modification of the
processing conditions may be required .Due to the wide variety of finishes
currently in use, compatibility should be tested on an individual basis.
2.
Exhaustion:
The chemical treatment exhausts rapidly from dilute aqueous solution onto
cotton. Adsorption is again improved at pH 7-8 .As in the padding application;
it is recommended that this treatment is followed by an acid fix and water
rinse. The spraying of solutions of antimicrobial active agents is not normally
recommended, due to the risk of the production and subsequent inhalation of
droplets of respirable size. Nevertheless, the treatment can be applied by
spraying, provide, suitable containment facilities are available; this method is
particularly suitable for non-woven fabrics.
Chito-oligo
saccharides as antimicrobial agents for cotton
Cotton fabric with good antimicrobial activity and durability to
washing is obtained by using chito-oligosaccharides without the need for a
binding chemical as a crosslinker. The fully deacetylated chitosan is
depolymerized into chito-oligosaccharides using sodium nitrite. The average
degree of polymerization (DP) of chito-oligosaccharides is determined by
calorimetric titration of a terminal aldehyde group of chito-oligosaccharides.
In a pad-dry-cure process, two different chito-oligosaccharides (DP = 3 and 10)
are applied to cotton fabric using the chemical reactivity of the terminal
aldehyde group. The antimicrobial activity and durability to washing of the
treated cotton are evaluated. The results show that at the fiftieth wash cycle,
the cotton fabrics treated with 2.4% chito-oligosaccharides are able to maintain
95% (for a DP of 3) and 100
Pre-treated
cotton/polyester fabric
Cotton/polyester fabrics containing cross-linked polyethylene
glycols (PEGs) show antimicrobial activity against a diverse group of bacteria
and fungi. The PEG-treated fabrics have substantial resistance to most
microorganisms relative to untreated fabrics. Because the level of formaldehyde
is extremely low, it is hypothesized that the antimicrobial activity of the
modified fabrics is due to a unique combination of physical and physicochemical
effects. These may include the hydrophilic nature of the cross-linked PECs that
desiccate microbes and deprive them of needed moisture and/or absorption and
release of latent heat by the bound PEG. However, the most probable effects that
impeded microbial growth may be attributable to the surfactant like properties
of the bound PEC, which disrupt cell membranes due to the dual
hydrophilic-hydrophobic characteristics of the PEGs.
Most finishing
methods directly use biocides in the finishing solutions and the biocidal;
function is achieved by controlled release or consuming the biocides combined on
the fabric. Therefore the imparted function will diminish as soon as the
biocides vanish from the fabrics, yielding a nonregenerable finish. The
traditional method has been an obstacle to achieving durable functional fabrics.
In the new process, precursors of biocidal compounds were utilized instead of
the biocides themselves in the treatment of cellulosic materials, and covalent
bonds established between the agent and fibres before the biocidal sites are
activated. The chemical structure ands of the finished fabrics and and their
relationship with the properties have been explored. In this section chemical
structures of MDMH treated fabrics are discussed. Structure property
relationships
analyzed.


The chemical
structure can be analyzed by:
·
Identification by FITR
·
Nitrogen analysis
·
Chlorine analysis
Chemical identification by FITR: Hydantoin compounds have two prominent bends at 1720 and 1770
cm-1. Thus bends at these wavenumbers can be used as indications of the
hydantoin structure grafted on to the fabric. Intensities of bend vary according
to the concentration difference of MDMH in the finishing baths. The
characteristics of the band are consistent with the structures of the products,
providing direct evidence of the structures, and can be used to quantitavely
analyze grafting rates of the chemical treatment.
Nitrogen analysis
The chemical
structures of treated and chlorinated fabrics can also be characterized using
elemental analysis, such as nitrogen and chlorine. Pure cotton fibres are mainly
cellulose and carbohydrates, containing undectable amounts of nitrogen. Thus
nitrogen contents of treated cotton fabric can be used as reference for the
amount of hydantoin groups grafted into cotton fabrics.
Chlorine analysis
After chlorine
bleaching, the treated fabrics will contain active chorine in the halamine form.
The active chlorine bonded on nitrogen, a halamine bond, is extremely polar in
the covalent bonds possessing partially positive charge. It is this chlorine
that can oxidize man organic structures in proteins or in some organic compounds
resulting in the inactivation of microorganisms. After the oxidation, the
chlorine atom is reduced to chloride and the halamine bond reverts to a N_H
bond. Because of the oxidative ability of the chlorine, its content on the
fabrics can be analyzed by using the iodometric titration method .The chlorine
contents on cotton and polyester/cotton fabrics treated with three different
concentrations of MDMH varied after repeated washing not only relying on the
concentrations of of MDMH in the finishing baths. (Graph below)
The Biocidal
properties of the fabrics are achieved by two steps of chemical processing
including finishing under acidic conditions and rinse with chlorine bleach. Each
of the process may have its on impact on physical properties of the fabrics.
Thus whether how much the treated fabric can retain its original tensile
strength at different finishing conditions is important. Factors, which may
affect the mechanical properties of the fabrics, are: Finishing
concentrations PH value Chlorine
concentration Drying conditions Effect of finishing concentrations: The amount or concentration of chemicals on the materials or
so-called add-on rates, will certainly affect the effectiveness of the function.
A higher concentration of finishing bath help to achieve a better adds on rate
on the fabrics thus improving the add-on properties. However, it also damages
the tensile strength of the material more substantially than lower
concentrations of finishing bath do. The graphs below show this effect on pure
cotton and polyester cotton blend
tensile strength retention of
tensile strength retention of
polyester/cotton blends treated
with MDMH
pure cotton treated
with MDMH
Thus polester cotton
blend having 35% cotton are less vulnerable to acidic conditions and
concentrations of finishing conditions Effect of pH values of finishing bath: The finishing of MDMH on cotton fabrics is carried out under low
pH conditions. Cellulosic structures are unstable to this condition because of
rapid decomposition of the chains catalyzed by the low pH. Following table shows
effect on fabric with varying pH concentrations.
As pH values
increase the physical properties of the fabric improve considerably. Effect of chlorine concentrations Chlorine solution being strongly oxidative
serves as the activating and regenerating agent in treatment. Chlorine was
considered very harmful to the functional moieties on the cellulose. Thus
chlorine concentrations were varied to examine the influence of chlorine damage
to the treated fabrics
Antibacterial Test Methods. Methods for
evaluating the effectiveness of an anti-bacterial finish on fabrics have been
the subject of much debate. Nevertheless, industry standard methods have been
developed by bodies such as AATCC, in the US, and SEK in Japan 8.9 Example of
this method are the AATCC 100 and the SEK
bacterial count methods. AATCC
test Method 100:
Four pieces of staked circular fabric swatches 4.8 + 0. I cm (about one gram)
are inoculated with O + 0. 1 ml of inoculum in a 250 mL jar. The inoculum as a
nutrient broth culute contains over I.O X 10^6 clone forming units (CFU) of
organism. After the swatches are inoculated, they are neutralized by 100 ml Of a
0.2% sodium thiosulfate solution in the jar. The contact time was the interval
between inoculation and neutralization. The jar is vigorously shaken and the
neutralized solution is diluted in serial. The dilutions, usually 10^0,10^1,
10^2 are plated on nutrient agar and incubated for 18-24 hours at 340C. The
numbers of bacteria recovered from the incubated finished fabrics are counted
and compared with that of untreated fabrics. SEK bacterial count : In this method 0.2 mi of a S. auresu suspension is inoculated
onto a 2 cm square of test fabric, and incubated at 370 for 18 hours 20 min
neutralizer solution is added, and the surviving bacterial counted using a
serial dilution pour plate technique. This method is considered to be the most
representative of a garment or
bedding
exposed to perspiration and bacterial contamination. In this test, a large
bacterial inoculum was used typical of that which has been found for example on
socks. Antifungal
Test Methods The assessment of
the antifungal activity of textiles is also by an agar plate method. A
suspension of fungal spores is inoculated onto the surface of an agar plate, the
test material placed on the agar, and further inoculum distributed over the
material. The samples are then incubated, and the surface area of fabric covered
with fungal growth visually
assessed. A " zone of inhibition" may be observed if the antifungal active agent can
leach from the fabric into the textile. A typical method is AATCC Test method
30. Assessment of durability It is essential that the antimicrobial effect last throughout the
normal lifetime of the product, which has been treated. The durability required
will vary, according to the lifetime of the article, exposure and washing
conditions. The tests has been based on two types of cycles: 1.Domestic wash cycle
Synthetic
detergent 4.5g/l Sodiurn
metasilicate 3.1g/l Hypochlorite
bleach 1.5g/l Wash at 45C, 15 min., rinse at 40C, and 10 min. Tumble dry at
800C, 12 MIN 2.Professional wash cycle Synthetic detergent
4.5g/l Hydrogen peroxide
3.1g/l Sodium percarbonate
1.5g/l Wash at 80C, 15 min. Rinse at 40C, IO min. Tumble-dry at 80C, 12
min. Each wash cycle considered as 5 washes in practice.
Anti bacterial
effect after domestic washing cycle
It is very common to find cotton or cellulose textiles with odors,
particularly if they are kept in a damp or soiled condition. For example a towel
will develop a musty, mildew odor if left damp or in a humid environment such as
bathroom. Clothing may develop characteristic foot or body odors .in the
majority OF vases these odors are generated by the action of microorganisms such
as bacteria or fungi. microbial odor generation on cotton or cellulosic textiles
can e controlled by treating the textile with a finish that in=impacts a durable
antimicrobial activity. The characteristic body odor is thought to be from 3-methyl-2
hexanoic acid produced by the species of Staphylococcus epidermis and
Corynrbacterium sp. Foot odor is quite different, and contains low molecular
weight carboxylic acids. This may be because different microorganisms prefer the
warm, moist conditions around the feet. Other odor molecules such as aldehydes
and amines may also be generated .A widely studied system is the break down of
urea into ammonia, by bacteria which secrete urease enzymes. This is of
particular importance in babies’ diapers, where ammonia generation gives a
pungent odor and also an increase indicated in nappy rash.
An investigation has been carried on poly (hexamethylene biguanide
hydrochloride)(PHMB), the basis of Reputex 20, an antibacterial that does appear
particularly suitable for cotton and cellulosic textile. It has been used for
some tears in a variety of antibacterial applications with high human contact,
such as swimming pool sanitization and preservation of personnel care products
However it is recently only that applications of this molecule on textile
industry have been developed. It is a relatively safe molecule and has low mammalian toxicity.
It shows good antimicrobial activity against broad spectrum of bacteria, yeasts
and fungi, and has a low environmental impact since it contains no heavy metals,
formaldehyde, organic halogens
15

16
EFFECT
OF ANTIMICROBIAL FINISHES ON MECHANICAL PROPERTIES

17

 Based on
concerns of chlorine damages to the Biocidal properties, lower concentration is
preferred in both activation or regeneration process.
Based on
concerns of chlorine damages to the Biocidal properties, lower concentration is
preferred in both activation or regeneration process.18
ASSESSMENT OF
ANTI-MICROBIAL FINISHES
19

20
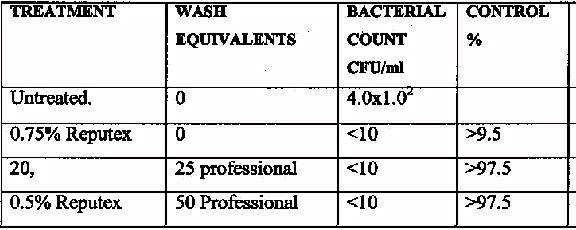
21
ANTIODOR FINISHES
22

The following tables
show observations of some experiments carried out.

.
There are two methods commonly used to reduce the spread of
microorganisms. Repellent finishes are popular in consumer goods because they
allow the fabric to “breathe” by allowing the passage of air through the
fabric while preventing the fabric from getting wet. Although this method has
shown a reduction in the transmission of microorganism, some may still be
transferred through the fabric or be transmitted from one area to another. Hence
the other method is to treat the fabrics with antimicrobial finishes that kill
microorganisms or inhibit their growth if microorganism comes in contact with
the fabric surface or if they are transmitted through the fabric. Therefore the
presence of live microorganisms is reduced, resulting in reduction of
microorganism transfer.
Application
of chemical finishes
Chemical finishing steps involves application of chemical solution
by padding, removing of water, curing the fabric after drying .The fabrics are
exposed to solution containing the antimicrobial finishes using Cromax
laboratory padder. The roller pressure is set to 60psi. Each fabric is padded
twice to ensure even distribution of the solution.
Triclosan is chlorine phenoxy compound that interrupts cytoplasmic
membrane and interfere with the metabolic functions of cell. However it is
incapable of rupturing the cell membrane of thicker blood cells, so it is safe
for human and animal contact.
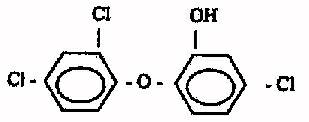
triclosan
REFERENCES
1. A
New Durable Antimicrobial Finish For Cotton Textiles
American Dye stuff Reporter; May 1996 (26-30)
2. Industrial
Antimicrobial Agents
Encyclopedia Of Chemical Processing; Volt 13; 1965 ;(223-253)
3. Durable
And Regenerable Antimicrobial Finishing Of Fabrics: Biocidal
Properties; Textile Chemists And Colorists; Vol 30; June 1998; (26-30)
4. A Durable
Antiodor Finish For Cotton Textiles
Textile Chemists And Colorists; Vol 28; May 1996; (28-300)
5.
Preparing Chito-Oligosaccharides As Antimicrobial Agents For Cotton
Textile-
Research-Journal. 1999; 69(7): 483-488.
6.
Antimicrobial textiles; ITB International Textile Bulletin 5/2000
7. Durable
And Regenerable Antimicrobial Finishing Of Fabrics: Biocidal
Properties; Textile Chemists And Colorists; Vol 31: May 1999
8.
Durable And Regenerable Antimicrobial Finishing Of Fabrics:
Chemical
Structure;
Textile Chemists And Colorists; Vol 30; June 1998; (31-35)
9.
Durable And Regenerable Antimicrobial Finishing Of Fabrics: Fabric
Properties; Textile Chemists And
Colorists; Vol 30; June 1998; (21-24)
10.
One-Bath Application of repellent and Antimicrobial Finishes to Nonwoven
Surgical Gown Fabrics.; Textile Chemists And Colorists; Vol 31:
March
1999; (11-16)
11.
New approach for imparting antibacterial activity to cellulose-containing
fabrics;
COLOURAGE; July 1998;(13-19)