Photographic Chemistry
Photographic chemistry is the true science of photography. Many people consider photographic chemistry strictly as the developer, fixer, stop, bath etc. I would like to submit to you that it goes further than this. Photographic chemistry also includes the film and paper emulsions as well. After all the changes that take place in a film or paper emulsion during the act of processing are chemical changes. The Silver halides in the emulsion are reduced to metallic silver, this is (if I recall my basic chemistry correctly) an endothermic process as it is breaking the bonds between the Silver and the Bromide, or the silver and the Iodide, or the silver and any of the other elements incorporated into the compound to create the halide.
What's the point?
I am sure you are asking "why do I care about photographic chemistry?" Well there are some good reasons. First knowing how to mix your own
chemistry from scratch will save you money in the long run. Instead of buying and mixing a gallon of Dektol at a time, and having half of it
go bad before you can use it, you can mix up a quart at a time. The pre-packaged developers usually run about $8.00 a gallon, you can make a gallon
for literally pennies on the dollar. You can adjust the amounts of the individual chemical compounds in the developer to achieve very different and dramatic results
on your film and paper. You can use developers no longer commercially available, such as D-23, and D-8. Or you can make developers that were never commercially
available, such as Beers variable contrast paper developer or D2D divided film developer. Best yet you can even make up your own developers.
Finally knowing how the developer works and whay it acts the way it does will help make you a better darkroom practitioner.
Film
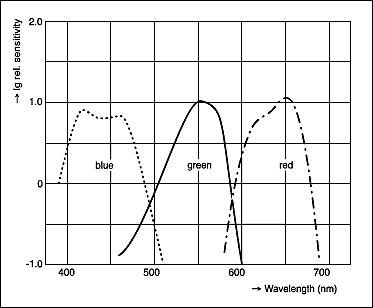
Film chemistry is quite a complex critter, but I will relate the small amount I know. Black and white film (and all film for thet matter) contains an emulsion, or suspension, of silver salts in a gelatin base. This gelatinous suspension is coated onto various substrate materials to give them a strong support. Sometimes this substrate may be a piece of plastic, a piece of paper, a canves or even a piece of wood. In the early days of photography it was usually a sheet of glass coated right before exposure in a darkroom or even a dark tent. Usually these "plates" were exposed while still wet. The silver halides in the emulsion are sensitive to light of pre-determined wavelengths. Almost all film is inherently sensitive to blue light. Films that are sensitive to only one narrow bandwidth of the spectrum is referred to as Orthochromatic, films that are sensitive to all of the visible spectrum are referred to as Panchromatic. When a film emulsion is produced the emulsion is allowed to "cook" or let the crystals grow to their optimum size neither too small and especially not too large. In this growth stage dyes are added to the emulsion to change the spectral absorption characteristics of the silver halides. Depending on the type of dye used or the amount introduced the film will be sensitized to the desired bandwidths of light wavelengths. Light is measured in Nano-meters. This refers to the length of the light wave. So if we refer to a film as being sensitive to the 400nm area of the spectrum then that means it is designed to catch that wavelength. Below is a set of spectral sensitivity curves for a given panchromatic film.
There are many other elements of photographic film besides the emulsion. there is the overcaot that protects the otherwise easily scratched emulsion from damage. There is usually a filter over the emulsion. There is an antihalation coating under the emulsion that keeps light rays from bouncing off the substrate onto the back of the emuksion and causing little diffuse ringlets of dispersed light from re-exposing the silver and creating a ghost like halo effect. There is a chemical additive in the emulsion that reduces a chemical halation effect. And there is the anti-curl coating that keeps the film from severely curling during drying. I hope you see now why I feel film is also a matter for chemistry discussion.
The process chemicals
Let's start this section with it's logical starting point, the developer. Developer is a chemical solution that reduces the silver halides in the film emulsion to
a metallic silver, it does so by a reduction action, or by breaking down the silver halides. Only the silver halides that have been exposed to light are reduced however,
and then that reduction is also based upon the amount of light they were exposed to, the more light absorption that has taken place the more reduction takes place.
In other words if a silver halide receives more light then it is turned into denser grains of silver during the reduction process. This is why a processed piece of film is
referred to as a negative, it is an exact opposite of the scene photographed, light areas are dark on the negative and dark areas are light on the negative, this is because
light areas in the scene reflect more light for the film to absorb. This concept is exactly what makes photography work. A negative image will reproduce on the paper as an exact duplicate
of the scene.
Now back to the developer. Developer incorporates different chemical compounds to do it's job. Some developers may have as few as three compounds and others can have a dozen or so.
One of the most popular of all developers is Kodak D-23 it contains three compounds and one of those is water. The most important component of any developer is the developing agent.
The developing agent is the chemical that actually reduces the silver halide. There are too many developing agents to list here but the most common are hydroquinone,
Kodak Elon (also known as metol), and Phenidone. When hydroquinone and metol are combined we refer to the resultant developer as an MQ developer. Likewise when phenidone and
hydroquinone are combined we refer to the resultant developer as a PQ developer. developing agents have a property referred to as potential. A fast acting developing agent reacts with the
silver halides much more aggresively and is said to have a higher potential. While a slow developing agent will be said to have a low potential.
The photographic developer also contains a chemical preservative, yep just like a twinky does. The preservative keeps the developer from dying a quick death due to the presence of free oxygen in the developer.
Too much oxygen in the developer will destroy it's usefullness by changing the chemical composition and the pH of the developer. Therefore we add a chemical that can readily absorb this
free oxygen through chemically bonding with it. Since overly robust mixing, and the addition of the water itself introduces free O2 into the solution it is important to add the
preservative. The preservative also eliminates staining that would occur from too much oxygen in the solution. The most common preservatives are sodium sufite, and sodium bisulfite.
Now we come to the accelerator. the developing agent left to itself is not active enough to develop the film in a reasonable amount of time, in fact some could take days to achieve the
desired result. This means we must speed up the activity with an accelerating agent. The accelerator actually does two things. First It causes the emulsions gelatin to swell and allows the
developing agent to penetrate the emulsion more quickly. The second action the accelerator performs is to neutralize the salt portion of the silver halide as it is released from the silver compound.
The pH of the accelerator changes the activity of the developing agent. the higher the pH the more active the developer, and the more active the developer the larger the grain structure of the film
will be. therefore the more acidic a developer is the less grain it will produce. the most widely used accelerators are Borax, Sodium metaborate, sodium carbonate, and even sodium hydroxide.
Finally the developer must be controlled yet again. Even the mildest of accelerators on their own will deliver too drastic a result and cause the developer to act too quickly, usually in a matter of a couple of
seconds, on the film. Times this low will not allow the photographer to achieve repeatable results. therefore we must add a final compound to our developer. This compound is known as a restrainer.
the restrsainer helps to buffer the activity of the accelerator and allows the photographer to work with more realistic developer times. The second thing a restrainer does is controls the activity of the
developing agent on the film emulsion by allowing it to penetrate deeper into the emulsion. If the restrainer was not used the developing agent would work only on the surface of the
emulsion and create chemical fog. the most common restrainer is Potassium bromide.
Now let's talk about the next step in the black and white process, the fixing bath. the fixer is basically a clearing bath. It takes all of the silver halides that were not exposed and therfore not reduced
and removes them from the film. It also clears the film of all other nonessential chemicals left in the emulsion. Finally most fixers incorporate
a hardener that makes the emulsion tough and more resillient to scratches.
Other Chemicals
Many photographers use a stop bath in between the developer and the fixer to halt the development action. I personally do not see any reason for this step unless you have an exceptionally active
developer. Most stop baths use acetic acid as the active ingredient. Since most fixers use acetic acid in them the use of a stop bath can be eliminated in most cases. The fixer acts as a great and
reliable stop bath in its own right. One thing to do when processing is to start emptying the developer from your daylight tank 15 seconds before the end of the development time.
The developer left on the film will continue processing the film for this period of time and then the fixer can go in right after to halt the developer action. If, however, you have a very active developer
by all means use a stop bath, since as little as five seconds can make a huge difference in the outcome of your film.
Hypo clearing agents are good to use when you are interested in archival processing of your film. Hypo clearing agents help to remove the fixer from the film during washing. Since fixer can cause film to stain if it is
not removed entirely the hypo clearing agent will help to eliminate this.
Photo-flo is a chemical solution that helps the film to shed water more easily during the drying process and is used between the washing and drying stages of development. The reason you want to eliminate
water before drying is to keep the film from having water spots on it once it is dried. This avoids any cleaning of the film prior to printing and avoids potential scratches.