
| home | games | about | mpjournal | school | links |

Mathematicians are fascinated by soap films because they can help to solve complex problems of space very simply.
Because of the tension within the liquid, a soap film will always from the smallest surface area between points or edges. A film stretched across a hoop , for example, will form a flat disc , and a film around a volume of air will form a sphere (a bubble).
When shapes or group of points are dipped into soap and lifted out again the information is revealed instantly.
Many types of curves, such as the parabola and the catenary curve, occur in nature. Scientists studying these curves have found that they can offer mathematical solution to given problems. By understanding how a certain type of curve is formed, it is possible to reproduce the shape in manufactured structure. One surprising tool for exploring curves is soap film. A bubble, or a film stretching shapes will form the smallest possible surface area to enclose a given volume, when a film joining several points will show the shortest distance between those points . Chemists use soap bubbles to find out how atoms are arranged in some molecules.
Engineers, and town and road planners, use the mathematical of soap films in many of their analyses.
<go to top>
 What is the natural shape of a bubbles? To find out, you need some detergent or
soap, a piece of soft florist's wire, a pair of pliers, and a dish.
What is the natural shape of a bubbles? To find out, you need some detergent or
soap, a piece of soft florist's wire, a pair of pliers, and a dish.
Use the wire to make a cube with one or two wire handles (a bit like a Cubic frying pan!). Sink this in the soap solution, pull it out, and check the shape of the bubble formed. If you pass the cube through a simple ring carrying a flat film of soap, you can produce interesting effects. Adding glycerine to soap solution makes the bubbles last longer.
Then try the shape on the right, and any other interesting shapes that occur to you, and see what sorts of bubbles form.
The bubble surface squeezes in so as to give the maximum volume for a give surface area. If there are other constraints, like bits of wire that the bubbles stick to, this can vary the shape of the bubble. With large and multiple bubbles, there are lots of conflicting forces, but they still reach a quick balance.
Spiral bubbles? Not really, just spiral films, really, single-sided bubbles, if you like. You need a piece of thick copper wire, about 30 or 40 cm long, a cylinder about 2 cm in diameter, a pair of pliers, a piece of thinner copper wire, and some bubble solution. The photograph probably explains it more easily than words. Wind the thick copper wire tightly around the cylinder to make a sort of spring. Take the spring off the cylinder, and stretch the spring out until it is about 4 cm long. Trim off any rough bits of the wire, and then bend in each end so that it cuts across the centre of the cylindrical space, as shown in the photograph.
Wind the thin wire around one of these end pieces, and pull it through the centre of the stretched spring, before winding it around the other end. When you dip this into the bubble solution, a spiral bubble will be formed, joining the spiral to the central strand.
If you stop and think about it, the spiral bubble is no more surprising than
the flat bubble you get in a circle of wire. Once again, the effect of surface
tension is to make the surface area as small as possible, stretched between the
boundaries. Since the outer wire is spiral, the bubble must be spiral as well.
<go to top>
 You will need some copper wire in two thicknesses to make a shape like this,
where the thin piece of wire is able to slide freely along the Y-shaped piece.
You will need some copper wire in two thicknesses to make a shape like this,
where the thin piece of wire is able to slide freely along the Y-shaped piece.
Put a small knob or twist on the end of each of the arms of the "Y", because you will be turning this gadget upside down to lower it into a soap solution, and then lifting it out.
Watch what happens to the slide when you do this.
The idea of this demonstration is to let you see how surface tension tries to reduce the surface area of even a flat film. In this case, the only way it can do this is to pull the slider up the Y, but this only happens after you have lifted the whole gadget clear of the soap solution. Can you work out why that is?
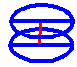 Cut two pieces of wire about 40 cm long, and bend them into two circles. Fit two
diameter wires at right-angles to each circle, and then join the two
"wheels" with a central shaft wire, so they are about 5 cm apart.
(When I tried this, I found two wires, twisted around each other, gave far
greater stability, and you need to either solder them or glue them with epoxy
resin.) If this contraption is dipped in soap solution, a cylindrical bubble
will be formed, bowing in around the middle. Experiment with different
distances: when you have found the best layout for the soap solution you are
using, soldering will make the whole arrangement much more stable.
Cut two pieces of wire about 40 cm long, and bend them into two circles. Fit two
diameter wires at right-angles to each circle, and then join the two
"wheels" with a central shaft wire, so they are about 5 cm apart.
(When I tried this, I found two wires, twisted around each other, gave far
greater stability, and you need to either solder them or glue them with epoxy
resin.) If this contraption is dipped in soap solution, a cylindrical bubble
will be formed, bowing in around the middle. Experiment with different
distances: when you have found the best layout for the soap solution you are
using, soldering will make the whole arrangement much more stable.
<go to top>
 This simple pair of demonstrations
will show an unusual side to
This simple pair of demonstrations
will show an unusual side to bubbles. Make a
loop of wire about 5 cm across, and add a handle to it, so you can dip the loop
into soap solution. Then tie a piece of cotton so it divides the circle roughly
in two, but so it remains a little bit slack. Use the illustration on the left
as your model for this.
bubbles. Make a
loop of wire about 5 cm across, and add a handle to it, so you can dip the loop
into soap solution. Then tie a piece of cotton so it divides the circle roughly
in two, but so it remains a little bit slack. Use the illustration on the left
as your model for this.
You can also make a section of the thread double, as you can see in the picture on the right.
When you take the first wire loop out of the soap solution, notice how the thread can move around freely in the soap film. Then burst the film on one side of the thread, and notice what happens next. See if you can work out what has caused this effect.
When you have dipped the second loop into the soap solution and pulled it out, burst the soap film between the two threads, and try to explain what you see.
The film of soap solution is elastic, pulling in all directions. When there
is film on both sides of the thread, there is no lopsided pull on the thread,
but as soon as you burst one part of the film, the remaining film pulls only in
one direction.
<go to top>