My Home Page ***Contact me***Printer-friendly [PDF] format
Study on the Possible
Effects of
Prolonged Consumption of
 Ethyl Alcohol on Insulin
Resistance
2002
A
DISSERTATION
submitted for the degree of
Doctor of Medicine
(General Medicine & Therapeutics)
to the
UNIVERSITY OF RAJASTHAN, JAIPUR
|
Guide:
Dr. Kailash Chandra  Jain,
               Â
MD, FICP, FIACM.
Professor,
Department of
Medicine,
RNT Medical College,
UDAIPUR (Rajasthan) 313 001.
|
    Â
Anirban Mitra
|
Dedicated to
The Patients
who, in their suffering,
had helped me in learning medicine
A word of honour
Words
can hardly express my deep sense of gratitude, indebtedness and reverence for
my esteemed teacher and guide Dr. Kailash Jain, MD, FICP, FIACM,
Professor, Department of Medicine, RNT Medical College, Udaipur who
inspired in me a spirit of dedication, precision and unbiased observation which
must be cornerstone of every scientific pursuit.
Without his constant help,
masterly guidance, advice, valuable suggestions, deep personal interest and
attention, this work would not have taken the present shape.
Anirban Mitra
Certificate
This
is to certify that Dr Anirban Mitra has carried out all the work in
connection with his dissertation entitled “Study on the Possible Effects of
Prolonged Consumption of Ethyl Alcohol on Insulin Resistance” under my direct guidance and
supervision.
The
work concerned with the study has been thoroughly carried out by the candidate
himself.
I
recommend this dissertation for the degree of “DOCTOR
OF MEDICINE” (General Medicine
and Therapeutics), to the UNIVERSITY OF RAJASTHAN,
JAIPUR.
Dr. Kailash Chandra Jain
MD, FICP, FIACM.
 ProfessorÂ
& Unit Head
Department of Medicine
R.N.T. Medical
College
UDAIPUR
(Rajasthan.)
Acknowledgements
I owe a deep sense of
gratitude to Dr. A.K. Gupta Principal and Controller, R.N.T. Medical College, Udaipur for allowing me to carry out the
dissertation work in this institution.
I express my sincere
gratitude to the esteemed Physician Dr. B.S. Bomb, Professor and Head of
the Department of Medicine, RNT Medical College, Udaipur for his constant
encouragement and support.
I am highly grateful
to Dr. L.K. Bhatnagar, Professor & Unit Head, Dr. Y.K. Bolya, Associate
Professor and Unit Head of Department of Medicine, Dr. S.K. Kaushik,
Professor and head Cardiology Unit for permission to include their patient in
this study.
Words can hardly
express my deep sense of gratitude to my esteemed teachers Dr. D.P. Singh, Associate
Professor, and Dr. P.S. Pipliwal, Assistant Professor, Department of
Medicine, R.N.T. Medical College, Udaipur, without whose guidance and profound
help this work could not have taken this shape. The abundant interest and
attention that they so keenly lavished upon this work has helped it to reach a
conclusion.
My sincere thanks to Dr.
D.C. Kumawat and Dr. Vijay Goyal, Associate Professors, Dr. Mahendra
Agarwal, Dr. Y.N. Verma, Dr. Mahesh Dave, Dr. R.L. Meena, and Dr.
Swati Shrivastava, Assistant Professors, Department of Medicine and Dr
D.C. Sharma, Assistant Professor Endocrinology, RNT Medical College, Udaipur
for their co-operation and guidance in carrying out this work.
I express my gratitude
to Dr. K.L. Mali, Professor, Department of Biochemistry, for permission
to use Clinical Biochemistry Laboratory for this dissertation.
I am extremely
thankful to Dr. Surendra Kumar Rajpurohit, Director and the technicians,
Mr. Khurshid, Mr. Anil, Mr. Yunus and others of Rajasthan Clinical Research
Centre for their laboratory assistance and guidance.
I am highly indebted
to my friends and colleagues, Dr. Raghu Prasad, Dr. Raghavendra S, Dr. Ajay Gainda,
Dr. Prem Choudhary, , Dr. Kiran Intodia, Dr. Sangeeta Bordia, Dr. Nagesh CM, Dr
Jayesh Gandhi, Dr. Bhanwarlal Bohra, Dr. Harish Sharma, Dr. Prabhat Kanwaria,
Dr. Guliver, Dr. Sushil Chauhan whose practical advice and apt comments
were often of great help in forming a perspective of the work.
It is impossible for
me to quantify and express in words my gratitude for my parents, Dr Rabi Mitra
and Srimati Ashima Mitra, and my sister,  brothers,
sisters–in-law, brother-in-law who are a constant source of inspiration and
encouragement in my life.
I will be failing in
my duty if I do not acknowledge the unlimited help extended by the nursing
staff of MB Govt. Hospital in carrying out this dissertation.                         Â
My
sincere thanks to all those people known and unknown who have by their
courtesy, enthusiasm and ardour helped me re-institute
my self-confidence and have been an invaluable aid in heralding the completion
of my dissertation.
Last but not the least; I extend my
heartfelt thanks to the patients whose consent for inclusion in the study was
instrumental in the culmination of my work.
Â
Dr Anirban
Mitra
TABLE
OF CONTENTS
|
Description
|
Page
No.
|
|
1.
Introduction
|
1
|
|
2.
Review of Literature
|
13
|
|
3.
Material and Methods
|
33
|
|
4.
Observations
|
43
|
|
5.
Discussions
|
56
|
|
6.
Summary and Conclusions
|
65
|
|
7.
Bibliography
|
68
|
|
8.
Miscellaneous
|
73
|
Introduction
Introduction
Diabetes Mellitus consists of a group
of metabolic disorders characterised by hyperglycaemia resulting from defects
in insulin secretion, insulin action or both. It is the commonest endocrine
disorder in human being. American Diabetes Association1
has classified diabetes mellitus into four major types and
many subtypes according to mechanism of production of hyperglycaemia. In type 2
DM, the condition may range from predominantly insulin resistance with relative
insulin deficiency to a predominantly insulin secretory defect with insulin
resistance.
Type 2 diabetes mellitus is rapidly emerging as a major public
health problem. There are estimated 86 million diabetics in Asia of which a
third (25-30 million) are in India.2Â Prevalence of diabetes mellitus (6.0 in
urban as compared to 2.8% in rural populations) was significantly (P<0.001) higher
in urban compared to rural subjects in a recent study3 conducted
in North India. In another study the age-adjusted prevalence rate was found to
be 8.2% in urban and 2.4% in rural population. 4 The
projected prevalence of diabetes in India by 2005 will be 30 to 35 million and
one out of every five diabetic subjects in the world will be Indian. 5 Studies in India in the last
decade have highlighted that not only is the prevalence of type 2 diabetes
high, but that it is increasing within the urban population. A recent national
survey of diabetes6
conducted in six major cities in India shows that the prevalence of diabetes in
urban adults was 12.l%. Prevalence of Impaired glucose tolerance (IGT) was also
high (14.0%). Several epiderniological studies by the Diabetes Research Centre,
Chennai have shown that the percentage of adult urban subjects affected has
increased from 5.2% in 1984 to 8.2% in 1989, 11.6% in 1995 and 13.9% in 2000. 7, 8 It is
calculated that in 2000 AD approximately about 33 million adults in India will
have diabetes.
In rural India the prevalence of type 2 diabetes, is 4-6 limes
lower than in urban areas8. Hence urgent action is required for proper detection
and management of diabetics in India including delineation of its causative
factors for adoption of proper long-term preventive measures.
Type 2 diabetes mellitus has a very complex aetiopathogenetic
sequence with both genetic and environmental influences playing its part. It
has a strong genetic component evidenced by high concordance rate (70 to 90 per
cent) in monozygotic twin and increased risk of diabetes in individuals whose
either parent is having type 2 diabetes mellitus (up to 40 per cent). Insulin
resistance is detected in many non-diabetic first-degree relatives of diabetic
persons. Exact genetic mapping of defects in type 2 diabetes mellitus is yet
not achieved. Moreover, genetic defects of insulin secretion or action may not
manifest unless another genetic defect (e.g. obesity) or an environmental event
is superimposed.
Type 2 diabetes mellitus is characterised by three
pathophysiologic abnormalities: peripheral insulin resistance, impaired insulin
secretion and excessive hepatic glucose production. Obesity, particularly
central, is commonly associated with type 2 diabetes mellitus, which further
augments insulin resistance. Initially there is a hyperinsulinaemic state with
normal glucose tolerance. Ultimately compensatory mechanism of β cells
fails and patient develops impaired glucose tolerance followed by frank
diabetes. The pathophysiology of type 2 diabetes, which accounts for over 90%
of patients with the disease, involves defects in three organ systems that conspire together to
produce abnormal glucose and lipid metabolism. While there is some uncertainty
regarding the primary lesion, or relative importance of different tissues,
metabolic defects in liver, peripheral target tissues such as fat and muscle
and pancreatic β cells all contribute to the syndrome. Insulin resistance,
which is defined as a state of reduced responsiveness to normal circulating
concentrations of insulin, is now recognized as a characteristic trait of type 2
diabetes, and contributes to abnormalities in all of these tissues. A number of
prospective epidemiological studies across several population groups have
indicated that type 2 diabetes progresses over a continuum of worsening insulin
action, beginning with peripheral insulin resistance and ending with a loss of
insulin secretion. In most patients, insulin resistance can be detected long
before the deterioration of glucose intolerance occurs. Insulin resistance is a
quite common state, associated with aging, a sedentary lifestyle, as well as a
genetic predisposition. The state seems to be fuelled by or perhaps to a
certain extent the result of obesity. The ensuing dysregulation of carbohydrate
and lipid metabolism that occurs as a consequence of insulin resistance further
exacerbates its progression. Beta cells of the pancreas normally compensate for
the insulin-resistant state by increasing basal and postprandial insulin
secretion. At some point, the beta cells can no longer compensate, failing to
respond appropriately to glucose. This ultimately leads to the deterioration of
glucose homeostasis, and the development of glucose intolerance.
Approximately 5 -10% of glucose intolerant patients per year
progress to frank diabetes, which continues to worsen as insulin resistance
increases. Adipose cells generate more fatty acids, the liver produces more
glucose in an unregulated fashion, and the beta cells undergo complete failure,
resulting in the late stages of the disease, where high doses of exogenous
insulin may be required. Even in the absence of diabetes, insulin resistance is
a key feature of other human disease states. Impaired insulin action coupled
with hyperinsulinemia leads to a variety of abnormalities, including elevated
triglycerides, low levels of HDL, enhanced secretion of VLDL, disorders of
coagulation, increased vascular resistance, changes in steroid hormone levels,
attenuation of peripheral blood flow and weight gain. Thus, insulin resistance
is often associated with central obesity, hypertension, polycystic ovarian
syndrome, dyslipidemia and atherosclerosis. This constellation of symptoms is
often referred to as Syndrome X, or Insulin Resistance Syndrome. Whether
impaired insulin action is directly responsible for all of the symptoms in
these patients remains unclear. However, the broad prevalence of insulin
resistance and its association with profound metabolic abnormalities is widely
accepted.
Insulin is the most potent anabolic agent known, promoting the
synthesis and storage of carbohydrates, lipids and proteins, and inhibiting
their degradation and release back into the circulation. Insulin maintains
glucose homeostasis by stimulating glucose uptake, utilization and storage in
muscle and adipose tissue, and inhibiting glucose output from liver. The
hormone also plays an important role in regulating lipid homeostasis,
stimulating lipogenesis in fat and liver, and inhibiting lipolysis in fat and
muscle. While the underlying cause of insulin resistance remains unknown,
investigations have focused on defects in signalling and metabolic pathways.
Studies in type 2 diabetic patients have shown that the primary
defect in insulin action lies in the regulation of glucose transport in adipose
and muscle. This process is mediated by the insulin-stimulated glucose
transporter9,
Glut4. Insulin action is
initiated through the binding to and activation of its tyrosine kinase
receptor. At the cellular level, the action of the hormone is characterized by
a diverse variety of effects, including changes in vesicle trafficking,
stimulation of protein kinases and phosphatases, promotion of cellular growth
and differentiation, and activation, or in some cases, repression of
transcription10.
Insulin resistance is caused by decreased ability of insulin to
act on peripheral target tissues (especially muscle and liver). This resistance
is relative as supernormal levels of insulin overcomes this resistance and
normalise plasma glucose. There is a rightward shift of insulin dose-response
curve along with reduced maximal response indicating 30 to 60% decreased
glucose utilisation11. There is decreased glucose
uptake and usage by insulin sensitive tissues such as muscles causing
postprandial hyperglycaemia as well as increased hepatic glucose output leading
to fasting hyperglycaemia. Glucose usage in non-insulin sensitive tissues (e.g.
brain) is not impaired.
Diabetes mellitus is commonly associated with systolic/diastolic
hypertension, and a wealth of epidemiological data suggests that this
association is independent of age and obesity. Much evidence12 indicates that
the link between diabetes and essential hypertension is hyperinsulinemia. Thus,
when hypertensive patients, whether obese or of normal body weight, are
compared with age- and weight-matched normotensive control subjects, a
heightened plasma insulin response to a glucose challenge is consistently
found. A state of cellular resistance to insulin action subtends the observed hyperinsulinism.
With the insulin/glucose-clamp technique, in combination with tracer glucose
infusion and indirect calorimetry, it has been demonstrated that the insulin
resistance of essential hypertension is located in peripheral tissues (muscle),
is limited to non-oxidative pathways of glucose disposal (glycogen synthesis),
and correlates directly with the severity of hypertension. The reasons for the
association of insulin resistance and essential hypertension can be sought in
at least four general types of mechanisms: Na+ retention,
sympathetic nervous system over-activity, disturbed membrane ion transport, and
proliferation of vascular smooth muscle cells. Physiological manoeuvres, such
as calorie restriction (in the overweight patient) and regular physical
exercise, can improve tissue sensitivity to insulin; evidence indicates that
these manoeuvres can also lower blood pressure in both normotensive and
hypertensive individuals. Insulin resistance and hyperinsulinaemia are also associated
with an atherogenic plasma lipid profile. Elevated plasma insulin
concentrations enhance very-low-density lipoprotein (VLDL) synthesis, leading
to hypertriglyceridemia. Progressive elimination of lipid and apolipoproteins
from the VLDL particle leads to an increased formation of intermediate-density
and low-density lipoproteins, both of which are atherogenic. Insulin,
independent of its effects on blood pressure and plasma lipids, is known to be atherogenic.
The hormone enhances cholesterol transport into arteriolar smooth muscle cells
and increases endogenous lipid synthesis by these cells. Insulin also
stimulates the proliferation of arteriolar smooth muscle cells, augments
collagen synthesis in the vascular wall, increases the formation of and decreases
the regression of lipid plaques, and stimulates the production of various
growth factors. Insulin resistance appears to be a syndrome that is associated
with a clustering of metabolic disorders, including non-insulin-dependent
diabetes mellitus, obesity, hypertension, lipid abnormalities, and
atherosclerotic cardiovascular disease.
Type 2 diabetes mellitus in Indians is in many ways different
in that of Caucasians. An analysis of epidemiological pattern of study
subjects in UK Prospective Diabetes Study 13 revealed that Indian patients were
younger (mean age 52.3, 47.0, 51.0 years respectively for Caucasians, Indians
and Afro-Caribbeans), less obese (BMI 29.3, 26.7, 27.9 kg/m2
respectively for Caucasians, Indians and Afro-Caribbeans). Indians had a greater
waist-hip ratio, lower blood pressure and lower prevalence of hypertension.
They were more often sedentary (39% as compared to 19% of Caucasians and 15% of
Afro-Caribbeans), more often abstained from alcohol (55% as compared to 21% in
Caucasians and 25% in Afro-Caribbeans) and had a greater prevalence of
first-degree relatives with known diabetes (36%, 44% and 34% for Caucasians,
Indians and Afro-Caribbeans respectively). Indians had more impaired insulin
sensitivity (19% of normal as compared to 27% in Afro-Caribbeans and 23% in
Caucasians) by homeostasis model assessment but less severely impaired
beta-cell function. (p value < 0.001 for all comparisons.
Another study14 showed
that Asian Indian men are more insulin resistant than Caucasian men independently
of generalized or truncal adiposity. The excessive insulin resistance in Asian
Indians is probably a primary metabolic defect and may account for the
excessive morbidity and mortality from diabetes and coronary heart disease in
this population
There is renewed interest in of insulin resistance (or
sensitivity) due to introduction of a new class of drug thiazolidinediones (glitazones).
These drugs exhibit their action by decreasing insulin resistance at cellular
levels acting on Peroxisome Proliferator Activated Receptor g (PPAR). They have shown excellent results
in many Indian diabetics. Hence other factors which may modify insulin
resistance need to be investigated and assessed.Â
Ethanol (C2H5OH) is a central nervous
system depressant that depresses activity of neurons, though some behavioural
stimulation is seen in low doses due to dis-inhibition of higher control.
Though alcohol drinking is an accepted social practice in many societies, it is
a common substance of abuse. Although alcohol consumption at mild to moderate
levels (one to two drinks a day) by otherwise healthy non-pregnant individual
has been shown to have some beneficial effect, especially cardiovascular,
alcohol in higher doses is toxic to most body system. An ethanol load in a
fasting healthy individual is likely to produce transient hypoglycaemia within
six to thirty six hours due acute suppressive effects of ethanol on gluconeogenesis.
This can result in glucose intolerance. However effect of chronic alcohol
intake on glucose intolerance or insulin sensitivity is less well defined15.
Rising trend of diabetes is becoming a major health problem and
matter of grave concern for health authorities, doctors, social workers and the
government. Parallel with this rising trend of diabetes, another disturbing rise
has been noticed in the trend of alcohol consumption in our country. With the
entry of multinational corporations in the market due to liberalisation coupled
with lax surveillance rules, the number of people taking to alcohol is
projected to increase.
Obtaining reliable prevalence of extent of alcohol abuse in India
is difficult because of differences in (a) definition; (b) survey instruments
used and (c) population screened between epidemiological studies. However
various studies16
indicate prevalence varying between 25% and 80% in general populations and 10%
to 58% amongst students. Another survey17 done by NIMHANS, Bangalore found almost a
quarter (23.3%) of the general hospital in-patients had associated drinking
problems which were more among medical than surgical in-patients. A study by Walia
M et al.18
in New Delhi showed a prevalence of alcohol consumption and/or smoking in 28.6%
of men with type 2 diabetes mellitus
A study19
was conducted on prevalence and social factors of alcoholism in rural areas of Ajmer
district in Rajasthan. The results showed that prevalence of alcohol abuse in
the sample was 24.7% (36.1% for males and 13.4% for females) and the percentage
of dependence was 3%. Alcohol abuse was found to be significantly associated
with religion (higher in Hindus with a relative risk of 8.65 in males and 5.21
in females), marital status (higher in married with a relative risk of 2.51 in
males), age (higher in age group more than 20 years with a relative risk of
2.50 in males and 1.63 in females), family structure (higher in nuclear or
joint families with a relative risk of 2.88 in males), educational status
(higher in illiterates with a relative risk of 1.53 in males) and occupational
status (higher in those engaged in agriculture with a relative risk of 1.43 in
males and 1.80 in females).In About 50% of both male and female users were
between 20 and 39 years of age; 8.1% of males and only 1.3% of females used
alcohol daily or several times in a week. Desi (country) liquor was the
beverage used by more than 85% of the users; 77.5% of males and 96.5% of
females consumed less than one quarter of a bottle of alcohol, and 65.3% of
males and 93.6% of females were taking alcohol at their houses only. Hence the quality of alcohol
offered to Indian public is a matter of concern to health authorities.
Prolonged alcohol consumption and its relationship with overt
manifestations of diabetes have interested scientists throughout the world.
Studies had been undertaken to explore this association in Japan20, Sweden21,
and Italy22.
Hence increasing alcohol usage may be one the “missing link” of
increasing insulin resistance in Indian population. Detailed study is warranted
to explore this association.
OBJECTIVES
The objective of the undertaken study was: -
1. To measure insulin
sensitivity in type 2 diabetics who are moderate to heavy drinker; who are
ex-drinker; alcoholics who are non-diabetics and a control cohort who are
non-diabetics and non-drinkers.
2. To compare insulin
sensitivity in above mentioned four categories
3. To attempt to find out
relation of anthropometric data such as Body Mass Index (BMI) and Waist-Hip
Ratio with insulin sensitivity in all four groups.
4. To assess effect of
alcohol on insulin sensitivity by comparing these four group of patients.
Review of Literature
Review of Literature
In India there is a rapidly escalating
epidemic of insulin resistance syndrome (diabetes, hypertension and coronary
heart disease). Contribution of genes and environment is under debate. Various
factors have been identified for increasing insulin resistance but exact
mechanism by which these factors act has not been elucidated.
Anthropometric measurements such as Body Mass Index (BMI) and
Waist Hip Ratio (WHR) give a rough estimate of Insulin resistance. Higher values
of these indices are usually indicative of decreased insulin sensitivity.
Various anthropometric indices have been used to quantify generalised and
central obesity. The WHR is the most widely used index of regional adipose
tissue distribution and is measured in a standing position. Waist circumference
is defined as the minimal circumference measured at the navel, and the hip
circumference is defined as the widest circumference measured at the hips and
buttocks24.
There is a well documented sex dimorphism in regional adipose
tissue distribution25. Indeed, despite the fact that women are
usually more obese as a group than men, male subjects more frequently
have significantly higher mean waist circumference and higher mean
WHR in agreement with the greater propensity of men to accumulate
excess fat within the abdominal cavity. Thus, the threshold values
suggested by Pouliot et al. 25 of 0.85 for women and 0.95 for
men are in agreement with those proposed in previous studies26. Since the WHR
has been shown to be associated, albeit moderately, with the amount
of abdominal visceral adipose tissue measured by CT or MRI [the
"gold standards" for such determination27], this index has
been widely used to investigate the relations between regional
adipose tissue distribution and metabolic profile. Thus it was
effective in predicting aberrations in glucose and insulin levels
and also showed a strong correlation between plasma lipids and blood
pressure. 28 WHR predicted subsequent diabetes in
men29
and coronary heart disease30 and was more predictive of these
endpoints than either the BMI or a more complex procedure using the
sum of multiple skinfold thickness.
Tai ES et al.31 compared body mass index (BMI), waist
circumference, waist-hip ratio (WHR) and percentage body fat measure as
predictors of fasting lipid profiles and insulin resistance in 109 Singaporean
Chinese. BMI was significantly correlated with insulin resistance and there
appeared to be a threshold at 26 kg/m2 above which the regression line became
steeper. WHR best predicted fasting triglyceride in both men and women. In
women, it was inversely correlated with high density lipoprotein cholesterol. A
similar association was seen in men but this did not reach statistical
significance. The investigators concluded that measurement
of BMI and WHR should be part of the assessment of cardiovascular risk in any
population as they connote different aspects of risk.
The association of insulin with cardiovascular disease (CVD)
may be mediated in part by the associations of insulin with CVD risk factors,
particularly blood pressure and serum lipids. These associations were examined
in 4576 black and white young adults in the CARDIA Study 32. Fasting insulin
level was correlated in univariate analysis with systolic blood pressure (r =
0.16), diastolic blood pressure (r = 0.13), triglycerides (r = 0.27), total
cholesterol (r = 0.10), high density lipoprotein (HDL) cholesterol (r = -0.25),
and low density lipoprotein (LDL) cholesterol (r = 0.14), and with age, sex,
race, glucose, body mass index, alcohol intake, cigarette use, physical
activity, and treadmill duration (all p less than 0.0001). After adjustment for
these covariates, insulin remained positively associated with blood pressure,
triglycerides, total and LDL cholesterol, and apolipoprotein B and was
negatively associated with HDL, HDL2 and HDL3 cholesterol, and apolipoprotein
A-I in all four race-sex groups. Higher levels of fasting insulin are
associated with unfavorable levels of CVD risk factors in young adults.
Walia et al. of RML Hospital, New Delhi conducted a
cross sectional study18
to find the prevalence of coronary risk factors in type 2 diabetes mellitus
patients and compared and correlated these risk factors in type 2 diabetics
with and without electrocardiographic and/or symptomatic evidence of coronary
heart disease (CHD). They found that mean age of patients was 53.12 year and
8.86 year was the mean duration of diabetes. 28.6% of the diabetic men were
found to be currently smoking and/or consuming alcohol, 82% were involved in
sedentary physical activity and 20.4% had family history of CHD. Central
obesity was observed in 46.7% of the cases; more so in females. 31.74% of cases
were hypertensive; more females than males had hypertension (33.8% vs. 30%).
Poor glycaemic control (HbA1c > 9.5%) was seen in 16.8% of the cases.
In about 52.5% of the total group hypertriglyceridemia was noted. Microalbuminuria
could be found in 35.93%. CHD was diagnosed in 15.57% of cases in this study.
Insulin sensitivity is measured by
various techniques. The hyperinsulinaemic euglycaemic glucose clamp
technique as described by DeFronzo et al.33 is the "gold standard"
for quantifying insulin sensitivity in vivo because it directly measures the
effects of insulin to promote glucose utilisation under steady state conditions. However, the glucose clamp
is not easily applied in clinical settings because intravenous infusion of
insulin, frequent blood sampling over a 3-h period, and continuous adjustment
of a glucose infusion are required for each subject studied. A well-accepted
alternative for estimating insulin sensitivity involves minimal model
analysis34 of a
frequently sampled IV glucose tolerance test (FSIVGTT). But this requires
obtaining approximately 30 blood samples over three hours. Furthermore,
although the minimal model index of insulin sensitivity (SIMM)
obtained by this method generally correlates with glucose clamp measurements,
identification of SIMM in subjects with impaired insulin
secretion (e.g. patients with diabetes) is often problematic. Hence need
for developing a simple and effective surrogate measurement was felt by many
investigators. Fasting insulin is presumed to be a surrogate measure of insulin
resistance. It had been used in few epidemiological studies as a measure of
insulin resistance. Another measure of insulin resistance devised by Matthews et
al. called “Homeostasis Model Assessment (HOMA)” depended
on relationship between fasting blood sugar and fasting insulin based on a
mathematical model. This measure had been used in various population trials
including UKPDS. According to this model Insulin resistance is measured by the
formula, HOMA-IR= G0 ✕
I0 /22.5 and β-cell function is measured by the formula,
HOMA-BCF= (20 ✕ I0)
/ (G0 – 3.5), where G0 is fasting glucose concentration
in millmole per litre and I0 is fasting insulin concentration in
µu/ml.
  Katz et al.36 of National Heart, Lung, and Blood
Institute, National Institutes of Health, Bethesda, Maryland devised a Quantitative
Insulin Sensitivity Check Index (QUICKI) based on fasting values of insulin
and glucose only and is obtained by the formulae
QUICKI =1 / [log10 (Insulin0)
+ log10 (Glucose0)]
where Insulin0 indicates fasting Insulin level and
Glucose0 indicates fasting glucose level.
They found that the index correlates well (Correlation
coefficient, r=0.78) with insulin sensitivity determined by euglycaemic clamp
method.
 Meta-analysis37 of studies of Insulin Resistance showed simple
mathematical combinations of fasting insulin and glucose measures (QUICKI)
provide estimates of insulin sensitivity with variability and discriminant
power comparable to those of euglycemic hyperinsulinaemic clamps and superior
to measures based on insulin alone. In accounting for both the glucose and
insulin levels, these measures are more generalisable to the full range of
metabolic conditions associated with insulin resistance. This underlies the
excellent correlations of these measures with clamps seen in obese and diabetic
insulin-resistant subjects. Furthermore, changes in insulin sensitivity can be
demonstrated using these tools in comparatively small groups of subjects.
Alcohol is known to cause hypoglycaemia in normal persons.
However, Christiansen C et al.38 showed that ethanol caused no change in
blood glucose or insulin concentrations in type 2 diabetics. The FFA level was
suppressed by ethanol while the triacylglycerol level was unaffected. The insulin
sensitivity as measured by euglycaemic clamp method was not affected by
ethanol. No major acute effect of ethanol on the glycaemic control in fasting
type 2 diabetic patients was found in comparison with what is seen in healthy
people
In experimental animals effect of ethanol on insulin release
has been measured. Patel and Singh39 found that ethanol inhibits glucose
mediated insulin release in perfused rat islet cells in a dose dependent
manner.
An important factor affecting glycaemic control in diabetics
who are alcoholics is poor diet and drug compliance. In a study40done amongst
in-city minorities in Los Angeles it was found drinking any alcohol-containing
beverage within 30 days was associated with poorer adherence to prescribed
dietary recommendations for the consumption of fibre (t = 2.4; P< 0.05), fat
(t = 4.2; P< 0.01), sweets (t = 2.7; P<0.01), and energy (calories) (t =
2.0; P< 0.05). Drinkers were also less likely to exercise for at least 20
minutes per day (t = 2.2; P< 0.05), comply with oral medication regimens (t
= 4.6; P< 0.01), or attend outpatient follow-up visits (r = -0.11; P<
0.05). Alcohol use did not significantly alter compliance with home glucose
monitoring, insulin use, or haemoglobin A1c levels, although there was a trend
toward higher haemoglobin A1c levels among drinkers (11.0 vs. 10.4).
Multivariate analysis of the data demonstrates that when demographic
characteristics, health care utilisation, and other diabetes-related variables
are held constant, the relation between alcohol use and dietary compliance
remained significant.
Various studies have shown conflicting results as far as effect
of chronic alcohol intake on glycaemic status and insulin resistance is
concerned.
In a randomised, multicentre, 12-month parallel open-label
study41
comparing the clinical safety and efficacy of insulin lispro with regular human
insulin, it was found that associated hypoglycaemia risk was lower with
increased alcohol consumption.
Solomon CG et al.42 assessed prospectively the association
between moderate alcohol intake and coronary heart disease risk in women with
type 2 diabetes mellitus, a group at high risk for cardiovascular disease. They
studied women in the Nurses' Health Study who reported a diagnosis of diabetes
mellitus at > 30 years of age. During 39,092 person-years of
follow-up from 1980 to 1994, there were 295 coronary heart disease events
documented among this population, including 194 cases of nonfatal myocardial
infarction and 101 cases of fatal coronary heart disease. Odds ratios derived
from logistic regression were used to estimate Relative Risks for coronary
heart disease as a function of usual alcohol intake, with adjustment for
potential confounding factors. Compared with diabetic women reporting no
alcohol intake, the age-adjusted relative risk for nonfatal or fatal coronary
heart disease among diabetic women reporting usual intake of 0.1 to 4.9 g
(<0.5 drinks) of alcohol daily was 0.74 (95% confidence interval = 0.56 to
0.98). Among those reporting usual intake > 5 g/d, it was 0.48 (95%
confidence interval 0.32 to 0.72) (P for trend <0.0001). Inverse
associations between alcohol intake and coronary heart disease risk remained
significant in multivariate analysis adjusting for several other coronary risk
factors. For alcohol intake of 0.1 to 4.9 g/d, the Relative Risk was 0.72 [95%
confidence interval 0.54 to 0.96] whereas for intake of 5 g/d or more the
Relative Risk was 0.45 [95% CI = 0.29 to 0.68]). The investigators concluded
although potential risks of alcohol consumption must be considered, these data
suggested that moderate alcohol consumption is associated with reduced coronary
heart disease risk in women with diabetes and should not be routinely
discouragedÂ.
Valmadrid CT et al. in their prospective population based
cohort study with a mean follow up period of 12.3 years conducted in Winconsin 43 found that
alcohol use was inversely associated with risk of Coronary Heart Disease(CHD)
mortality in older onset diabetic subjects. The CHD mortality rates for never
and former drinkers were 43.9 and 38.5 per 1000 person years, respectively,
while the rates for those with alcohol intakes of less than 2, 2 to 13, and 14
or more g/d were 25.3, 20.8, and 10.0 per 1000 person years, respectively.
Compared with never drinkers and controlling for age, sex, cigarette smoking, glycosylated
haemoglobin level, insulin use, plasma C peptide level, history of angina or
myocardial infarction, digoxin use, and the presence and severity of diabetic
retinopathy, former drinkers had a relative risk (RR) of 0.69 (95% confidence
interval [CI] 0.43- 1.12). For those who drank less than 2 g/d (less frequent
than 1 drink a week), the RR was 0.54 (95% CI, 0.33 0.90); for 2 to 13 g/d, it
was 0.44 (95% CI, 0.23 0.84); and for 14 or more g/d (about 1 drink or more a
day), it was 0.21 (95% CI, 0.09 0.48). Further adjustments for blood pressure,
body mass index, education, physical activity, diabetes duration, hypertension
history, overt nephropathy, peripheral neuropathy, lipid measures, or intake of
medications such as aspirin and anti-hypertensive agents did not change the
associations observed. These results suggested an overall beneficial effect of
alcohol consumption in decreasing the risk of death due to CHD in people with
older onset diabetes.
Relationships between alcohol use and Insulin Sensitivity Index
(SI) and cardiovascular disease risk factors were assessed by Bell et al.44 in a
cross-sectional analysis of 1,196 white, African-American, and Hispanic men and
women from the Insulin Resistance and Atherosclerosis Study (IRAS). Five
categories of previous-year alcohol use (never, <0.5 drinks/day, 0.5-0.99
drinks/day, 1-2.99 drinks/day, and > or =3 drinks/day) and log SI + 1
(frequently sampled intravenous glucose tolerance test with Bergman minimal
model analysis), log fasting insulin, log triglyceride, HDL cholesterol, and
systolic/diastolic blood pressure were examined using analysis of variance. In
this study, univariate analysis showed an inverse U-shaped relationship between
SI and alcohol intake, with a peak at the 0.5-0.99 drinks/day category. A
U-shaped relationship was observed between fasting insulin and the lipid and
blood pressure measures. After adjustment for demographic (clinic, sex,
ethnicity, age), lifestyle (smoking, dietary energy/fat intake, physical
activity), and physical (BMI, waist circumference) variables, the
alcohol/insulin association was attenuated, but the association with lipids and
blood pressure remained for high-intake categories. These data suggested that
the enhanced SI associated with light-to-moderate alcohol consumption might be
a function solely of a BMI and central adiposity profile more favourable to
higher SI.
Â
During Osaka
Health Survey20 in Japan, the
investigators enrolled 6,362 Japanese men aged 35-61 years who did not have
diabetes, impaired fasting glucose, hypertension, or liver cirrhosis at study
entry. Type 2 diabetes was defined as a fasting plasma glucose (FPG) level >
or =126 mg/dl or was diagnosed by a physician. Data on alcohol consumption were
obtained from questionnaires. The investigators confirmed 456 cases of type 2
diabetes during the 62,016 person-years of follow-up. It was found that the
relationship between daily alcohol consumption and the risk of type 2 diabetes
among lean men and among men with a higher BMI was paradoxical. Among lean men
(BMI < or =22.0 kg/m2), heavy drinking was associated with an increased risk
of type 2 diabetes. Men who consumed > 50.1 ml/day of alcohol had a
relative risk (RR) of 2.48 (95% CI 1.31-4.71) compared with non-drinkers after
adjusting for age, BMI, regular physical exercise, parental history of
diabetes, smoking habits, and FPG level. However, among men with a BMI >22.1 kg/m2,
moderate drinking (29.1-50.0 ml/day) was associated with a decreased risk of
type 2 diabetes. Daily moderate drinkers had a multiple adjusted Relative Risk
of 0.58 (95% confidence interval 0.39-0.87) compared with non-drinkers. It was
concluded that among men with a BMI > 22.1 kg/m2, moderate alcohol
consumption was associated with a reduced risk of type 2 diabetes, but among
lean men (BMI < 22.0 kg/m2),
heavy alcohol consumption was associated with an increased risk of type 2
diabetes.
Another population-based cross-sectional study21 consisting of
3,128 Swedish men, aged 35-56 years investigated the association between
alcohol consumption and impaired glucose tolerance and Type 2 diabetes
mellitus. Oral glucose tolerance testing identified 55 cases of Type 2 diabetes
and 172 cases of impaired glucose tolerance. Information on alcohol
consumption, family history of diabetes, smoking and physical activity was
obtained by questionnaire. After adjustment for family history, smoking,
physical activity and body mass index, the odds ratio of diabetes was 2.1 (95%
confidence interval [CI] 1.0-4.5) in men with high consumption of alcohol
(corresponding to over 12 drinks per week) and 0.7 (0.3-1.8) in moderate
consumers (7-12 drinks), compared to occasional drinkers. For impaired glucose
tolerance, the corresponding odds ratios were 0.7 (0.5-1.1) and 0.6 (0.4-1.0),
respectively. Separate analyses for type of beverage indicated that high
consumers of beer, spirits and wine had an odds ratio for diabetes of 2.9
(1.2-6.9), 3.3 (1.4-7.8) and 1.2 (0.5-2.7), respectively. The results indicated
that high consumption of alcohol increases the occurrence of Type 2 diabetes
and that this may primarily concern consumption of beer and spirits. For
impaired glucose tolerance, regular alcohol consumption was associated with a
reduced prevalence, particularly at moderate levels.
In a large prospective and cross-sectional study, called Bruneck
Study22
done in Bruneck, Bolzona province, Italy, relation between regular alcohol
intake and insulin sensitivity was assessed in 820 healthy men and women.  The
study showed that the fasting insulin concentrations were 12.4, 10.0, 8.7 and
7.1 µU/ml in non-drinkers, subjects reporting drinking of low (1-50 g/d),
moderate(51-99 g/d) and heavy (>100 g/d) respectively (p<0.001). The
changes were independent of sex, BMI, smoking, medication and diet. The
investigators concluded that low to moderate amount of alcohol, when consumed
on a regular basis, improves insulin sensitivity. Insulin was thought to be
intermediate in alcohol consumption and metabolic syndrome.
Singh et al.45 found the plasma insulin and C-peptide
responses to glucose were delayed in diabetic patients compared to controls but
were not affected by ethanol. In vitro, ethanol at a concentration of 100 mg/dl
or greater, significantly decreased insulin binding to erythrocytes in a
dose-related manner. Scatchard analysis of competitive insulin binding to
erythrocytes indicated that ethanol reduced insulin binding affinity but not
binding capacity.
Among the nondiabetic participants of the Kaiser Permanente
Women Twins Study46
(1989 through 1990), within the range of light to moderate drinking habits,
alcohol consumption was inversely related to fasting and postload insulin
levels. This relation did not explain associations of alcohol intake with lipid
levels and might instead reflected an additional mechanism by which moderate
alcohol consumption impacts cardiovascular disease risk.
Recently in a randomized controlled crossover trial47 of 63 healthy
postmenopausal women, conducted at a clinical research centre in Maryland
between 1998 and 1999, participants were randomly assigned to consume 0, 15, or
30 g/d of alcohol for 8 weeks each as part of a controlled diet. All foods and
beverages were provided during the intervention. An iso-caloric beverage was
provided in the 0-g/d arm. Energy intake was adjusted to maintain constant body
weight. A complete set of plasma samples was collected and analyzed for 51
women who completed all diet treatments. Consumption of 30 g/d of alcohol
compared with 0 g/d reduced fasting insulin concentration by 19.2% (p = .004)
and triglyceride concentration by 10.3% (p = 0.001), and increased insulin
sensitivity by 7.2% (P = .002). Normal-weight, overweight, and obese
individuals responded similarly. Only fasting triglyceride concentration was
significantly reduced when comparing 0 and 15 g/d of alcohol (7.8%; P = .03),
and no difference was found between consumption of 15 and 30 g/d of alcohol;
however, there was a significant linear trend (P = .001). Fasting glucose
concentrations were not different across treatments. Investigators concluded
that consumption of 30 g/d of alcohol (2 drinks per day) has beneficial effects
on insulin and triglyceride concentrations and insulin sensitivity in nondiabetic
postmenopausal women.
Kornhuber HH et al. 48 investigated the relationship between
alcohol consumption, the "liver enzymes" and other metabolic
parameters, including the serum lipids in 1214 adult persons. In 798 of the
persons, glucose tolerance tests with measurement of plasma insulin were performed
(young and old male and female adults, either
volunteers or patients without liver-related diseases). There was a high
correlation of the three transferases GOT, GPT and GGT not only with the
reported alcohol consumption but also with the plasma insulin. Most of the
insulin increase, however, occurred in that range of the three transferases
which has been considered to be the normal one. The C-peptide showed the same
behaviour. Plasma insulin was also raised in relation to overweight, but only
in persons with the sum of the three transferases over 30 U/l, not in persons
who did not drink alcohol and who had really normal transferases (sum of the
three transferases below 30 U/l measured at 25 degrees C). The quotient of
plasma insulin divided by the relative body weight (Broca Index) was constantly
low in the range of really normal transferases (up to 30 U/l), thereafter
rising significantly, but only in the range of the transferases considered to
be the normal one (SGOT to 17, SGPT to 22, GGT to 28 U/l, thus sum up to 67).
Serum glucose in the tolerance test also rose with the transferases but much
less than the plasma insulin. The correlation between both GGT and the sum of
the three transferases with the plasma insulin was significantly positive and
independent of the relative body weight. It was concluded that overweight
(which is generally believed to be the main risk factor for
non-insulin-dependent diabetes), and insulin
resistance (which leads to hyperinsulinaemia), are largely caused by the toxic
effects of "normal" daily alcohol, more in the human male than in the
female.
 Hyperinsulinaemia (which blocks lipolysis) is caused by a
toxic effect of ethanol and its metabolites, independent of caloric input and
overweight. Hyperinsulinaemia is at least in the human male at present,
probably the most important cause of obesity. In obesity, caused by
"normal" alcohol consumption, a vicious circle occurs: the
enhancement of the triglycerides and, consequently, the free fatty acids leads
to a further decrease of glucose utilisation by the muscles which ultimately
lead to diabetes.
It is known
that chronic alcoholics and type II diabetics show hyperlipidaemia,
characterised by hypertriglyceridaemia and in a minor degree by hypercholesterolaemia.
The mechanisms underlying the effect of ethanol and carbohydrates on plasma
lipids seem to be different; therefore in diabetic subjects, chronic alcohol
consumption could produce a more severe hyperlipidaemia and so accelerate
atherosclerotic events. In order to verify it Bertello PD et al.49 measured
plasma cholesterol, HDL-cholesterol, and triglycerides and investigated the
presence of micro- and macroangiopathy in two groups of non-insulin-dependent
diabetics, differing each other for daily alcohol intake (18 chronic male
alcoholics and 30 male subjects consuming respectively more than 150 g and less
than 50 g of alcohol daily). In alcoholics, no clinical features, laboratory
and echographic findings of cirrhosis and pancreatic disease were present. The
data were analysed by Student's "t" and chi-squared tests. No
significant differences could be detected (alcoholics/occasional drinkers,
means ± 1 SD) either in the plasma levels of cholesterol (181.7 ± 39.3/198.2 ±
32.5), HDL-cholesterol (43.4 ± 12.7/38.5 ± 11.9), and triglycerides (105.5 ±
56.4/159.7 ± 114.8) and in the frequency of micro (22.2%/16.6%) and macroangiopathy
(16.6%/26.6%) between the two studied groups.
Snehalatha et al. 23 performed a study to determine plasma
levels of proinsulin (PI) and specific insulin (SI) in normoglycaemic (NGT)
Asian Indians and to assess the effect of obesity and impaired glucose
tolerance (IGT) on these concentrations. Blood samples from 151 adult
non-diabetic South Indian subjects were collected during an epidemiological survey
of diabetes. Plasma SI and PI levels were measured in fasting and 30-minute and
120-minute samples of a glucose tolerance test (World Health Organisation
criteria) using monospecific antibodies. The total insulin (TI) level was also
measured by the non-specific assay. The molar ratio of PI to SI (PI/SI) was
calculated. Correlation of the peptides with anthropometry, serum lipids, and
blood pressure (BP) were studied by univariate and multivariate analyses.
Comparisons were also made in NGT versus IGT groups. As expected, TI values
were higher than SI values, but the patterns of response were similar for both.
SI and PI responses in NGT were similar to the values found in
Mexican-Americans who had a higher body mass index (BMI). Asian Indians were
thus found to have a high SI response despite a low BMI. Obesity and IGT
produced an increased response of both PI and SI, with normal PI/SI ratios thus
showing an absence of hyperproinsulinaemia in either condition. Fasting PI
showed a strong association with serum triglycerides, and proinsulin at 120
minutes was associated with cholesterol. None of the peptides showed a
correlation with Blood Pressure. Using specific assays for insulin and PI, it
was shown that Asian Indians with NGT have a hyperinsulinemic response despite
a low BMI. Obesity and mild hyperglycaemia in IGT produce a simultaneous
increase in PI and SI with no alteration in the PI/SI ratio.
Considering various studies and data, an expert committee
headed by RB Singh50
opined that moderate physical activity with the aim of burning 300 Kcal/day
(> 1255 KJ/day), and cessation of tobacco and alcohol consumption, may
provide an effective programme for prevention of diabetes and its vascular
complications in Indians.
Material and Methods
Material and Methods
Patients admitted in various medical wards
as well as attending Out PatientsÂ’ Department of Mahrana Bhopal General
Hospital Udaipur attached to RNT Medical College were included in this study
after taking an informed consent.
Four categories of patients were included in this study.
Inclusion criteria and exclusion criteria of these group was as below.
Group 1: type 2 diabetics who are alcoholics
Twenty five (25) patients who are
(i) having type 2 diabetes mellitus ,
(ii) aged more than 45 years,
(iii)on glycaemic control with oral hypoglycaemic agents,
(iv) consuming 7 or
more drinks per week (moderate to heavy drinkers) for more than five years was
included in this group. Exclusion criteria for this group was
(a) any past
history of ketosis and
(b) patient
requiring insulin for glycaemic control in recent past,
(c) evidence of
liver failure or cirrhosis.
Group 2: type 2 diabetics who are ex-alcoholics
Twenty five (25) patients were included in this group.
Characteristics of these patients were as following: -
(i)
Age 45 years or more.
(ii)
History of consumption of moderate to heavy amount of
alcohol (> 7 drinks per week) for at least 5 years but not consuming
alcohol for at least 6 weeks before inclusion in the study.
(iii)
Absence of liver diseases
Group 3: Alcoholics who are non-diabetics
 Twenty five (25) patients with history of intake of moderate
to heavy amount of alcohol (7 drinks or more per week) for more than five (5)
years with no history or biochemical evidence of diabetes (having fasting and
2-hour post-prandial plasma glucose less than 126 and 200 mg/dl respectively)Â
was included in this group.
Group 4: Control cohort who are non-diabetics non-alcoholics
Twenty five (25) patients suffering from unrelated illness
excluding liver disease with no history of type 2 or type 1 diabetes mellitus
and who had never consumed alcohol was included in this group. All the patients
were selected to match other three groups in other demographic characteristics
as well.
A detail history was taken to assign patients to corresponding
groups. In patients belonging to group 2 and 3, information was gathered
regarding onset and progress of diabetic symptoms and duration of diabetes.Â
Particular emphasis was given on history of addiction
especially alcohol intake. PatientÂ’s drinking patterns was ascertained by
questionnaire and was crosschecked with relatives where available. A rough
estimate of weekly alcohol consumption was made. History of other addictions,
especially smoking, was also taken.
A detail drug history assessing intake of various anti-diabetic
and other medications was made to ascertain any confounding factors while
comparing alcohol drinker with non-alcohol drinker groups. Compliance was
assessed by questioning patient and relatives.
A complete physical examination was done to find out any
clinical evidence of microangiopathic along with macroangiopathic complications
of type 2 diabetes mellitus. Height was measured in centimetres against a
calibrated wall in standing erect posture. Weight was measured using a standard
spring balance. Body Mass Index (BMI) was calculated from these measurements.
Waist girth was measured as the minimum circumference between iliac
crest and rib cage, and hip girth was measured at the maximum width
over the greater trochanters. Waist-Hip ratio (WHR) was calculated from these
measurements. Blood Pressure was measured using a standard mercury manometer in
sitting posture after rest for 5 minutes. An high BP was confirmed by two more
measurements
 Care was taken to detect evidence of hepatic dysfunction and those
cases was excluded from study.
Patients were then investigated. Routine examinations included
fasting and post-prandial plasma glucose, serum urea and creatinine, urine for
albumin, sugar, ketone and microscopy. Other routine investigations included billirubun,
SGOT, SGPT and Ultrasonography of abdomen for evaluation of hepatic function.
Plasma glucose was estimated by enzymatic method called GOD/POD
METHOD52.
Principle of this test is as below
Glucose is oxidised by the enzyme Glucose oxidase (GOD) to give
D-Gluconic acid and hydrogen peroxide. Hydrogen peroxide in presence of enzyme peroxidase
(POD) oxidises phenol which combines with 4-aminoantipyrine to produce a red
coloured quinoneimine dye. The intensity of colour produced is proportional to
the glucose concentration of the sample.
Method: Clean, dry test tubes were labelled as Blank (B),
Standard (S) and Test (T). In each of the tubes, 1.0 ml of prepared working
enzyme were added. To the test tube labelled B, S and T, respectively 10 µl of
distilled water, 10 µl of standard solution (containing 100 mg/dl glucose
concentration) and 10 µl of test serum were added. They were mixed well and
incubated at 37° C for 15 minutes. Then the adsorbance of the test (T) and
Standard (S) were measured against blank on a photocolorimeter with a green
filter at 505 nm.
Glucose in mg/dl = A of (T) /A of (S) x 100
Cholesterol and Triglycerides were measured by standard
enzymatic methods as described below using Stat Fax® semi-autoanalyser.
LIPID
PROFILE ESTIMATION BY SEMI AUTOANALYZER[STAT FAX 2000]
1. TOTAL CHOLESTEROL (Enzymatic method) 53
PRINCIPLE:
-Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Cholesterol
                                            Â
Esterase
    Cholesterol Ester + H2O
¾¾¾¾® Cholesterol + Fatty acids
                                Â
Cholesterol
                                Â
Oxidase
    Cholesterol + O2
¾¾¾¾® Cholestenone + H2O2
                                                                Â
Peroxidase
    2H2O2 +
Phenol + 4-Aminoantipyrine ¾¾¾® Red quinone + 4 - H2O2
The concentration of cholesterol in
the sample is directly proportional to the intensity of the red complex (Red Quinone)
which is measured at 500 nm.
2. TRIGLYCERIDES 54, 55
         PRINCIPLE: -        Â
                                         Â
LipaseÂ
      Triglycerides + H2O
¾¾¾¾® Glycerol + Free Fatty acids.
                                       Â
GKÂ
 Glycerol
+ ATP ¾¾¾¾® Glycerol - 3- phosphate + ADP.
                           Â
                        Â
                                                                                                                                                                                                                                                                                                                                              Â
GPOÂ Â Â
Glycerol - 3- phosphate + O2 ¾¾¾¾® DAP + H2O2.
                                     Â
                                            Peroxidase
H2O2
+ 4 AAP + DHBS ¾¾¾¾® Quinoneimine dye + 2 H2O
GKÂ Â Â Â Â Â Â
=Â Â Â Glycerol Kinaes
GPOÂ Â Â Â Â
=Â Â Â Glycerol Phosphate Oxidase
DAPÂ Â Â Â Â
=Â Â Â Dihydroxyacetone phosphate
ATPÂ Â Â Â Â
=Â Â Â Adenosine triphosphate
4-
AAP =Â Â Â Â 4 Aminoantipyrine
DHBSÂ Â
=Â Â Â Â 3,5- dicholoro-2-hydroxybenzene sulfonate
H2O2Â Â Â Â
=Â Â Â Â Hydrogen Peroxide
The absorbance of standard and each sample tube were read
against reagent blank at 510 nm.
Among the special investigations, fasting insulin levels was
determined by Radioimmunoassay (RIA) method using I125 tracer bound
antibodies in an approved laboratory. RIA is a structurally specific immunochemical
assay which measures Immunoreactive Insulin. It is an extraordinary sensitive
and specific which can measure concentration as low as 10-13 mole of
substance. 57
Sample collection and preparation: Serum or plasma
was used and the usual precautions for venipuncture were observed. Specimens
were stored at 2 to 8°C for up to 24 hours or frozen at Â20°C or lower for
longer periods.
Principles of the test:56,58
The procedure
follows the basic principle of Radioimmunoassay where there is a competition
between a radioactive and a non-radioactive antigen for a fixed number of
antibody binding sites. The amount of I125 labeled insulin bound to
the antibody is inversely proportional to the concentration of unlabeled
insulin present. The separation of free and bound antigen is easily and rapidly
achieved by using a double antibody system.
Assay Procedure: The assay was done using the DSL-1600
Insulin RIA kit manufactured by the Diagnostic Systems Laboratories, Inc., Webstar, Texas, USA.
1.
All
the assay reagents were allowed to reach room temperature (~25°C) after
removing from the refrigerator and mixed before use by gentle and thorough
shaking. After reconstitution of Standards and Controls, they were mixed
thoroughly, avoiding foam.
2.
Plastic tubes (12x75mm) were labeled and arranged for
Total counts, Standards, Controls, Non-specific binding (NSB) and samples were
studied in duplicate.
3. To the appropriate tubes 100 µl of
the Insulin Standards, Controls, and serum samples of the corresponding study
subjects were added. In NSB tubes 200 µl of the 0 µIU/ml Insulin Standard were
added.
4. In all tubes, except NSB and Total
count tubes 100µl of Insulin Antiserum were added.
5. 100µl of Insulin I125
reagent were added to each tube.
6. All tubes were vortexed.
7. Tubes were incubated at 2-8°C for
16 hours.
8. To all tubes except Total Count tubes
1 ml of precipitating reagents were added after being shaken thoroughly.
9. All tubes were vortexed.
10. Tubes were incubated at room
temperature (~25ºC) for 10 to 15 minutes.
11. All tubes, except Total count
tubes, were centrifuged at 1500 ´ g for 20 minutes.
12. All tubes, except the Total count
tubes, were decanted by simultaneous inversion with a sponge receptacle. They
were allowed to be drained on absorbent material for 15-30 seconds and were
gently blotted to remove any droplets adhering to the rim before returning them
to the upright position.
13. All the tubes were counted in a
gamma counter for one minute.
14. Mean counts per minute (cpm) were
calculated for each Standard, Control and unknown serum samples. Mean counts
per minute for non-specific bindings were subtracted from all counts to obtain
corrected results. For each Standard, Control and test serum sample % B/B0
was calculated as
|
% B/B0 =
|
Mean Sample counts - NSB
counts
|
X 100
|
|
Mean
cpm of zero Insulin Standards - NSB counts
|
Â
15. A curve of % B/B0 for each Standard
against the insulin concentration was plotted on a semi-log graph paper. A
standard curve was drawn through the means of duplicate points. From this standard
curve, concentrations of test and control sample were determined
After measuring serum Insulin, Quantitative insulin
sensitivity check index and HOMA indices was calculated from the formula
QUICKI= 1/ [log10 (Insulin0)
+ log10 (Glucose0)]
HOMA-IR ={Insulin0 X(Glucose0/18)}/22.5
HOMA‑BCF= (20XInsulin0)
/ {(Glucose0/18) – 3.5} where Insulin0 fasting insulin in
µu/ml and Glucose0 is fasting glucose in mg/dl
After collection of data they were tabulated.
Statistical analysis was done including t-test for comparison among all four
above mentioned groups. Various confounding variables were matched. Assessment
of surrogate markers of insulin resistance such as Body Mass Index (BMI),
Waist-Hip ratio (WHR) and Serum Triglyceride were done by comparing with the
measured value of Insulin Sensitivity Index.
By comparing all four groups amongst themselves by
t-test, effect of alcohol consumption on insulin resistance in diabetics as
well as non-diabetics was ascertained. Correlation between various parameters
was determined by PearsonÂ’s univariate regression analysis and multivariate
regression analysis.
Â
Observations
Observations
In this study, patients admitted in various wards and attending
the OPD of Maharana Bhupal Government Hospital were included. Clinical
examination, anthropometric measurements and bio-chemical assay was conducted
in patients belonging to each of four mutually exclusive groups, Group A: Type
2 diabetic alcoholics. Group B: Type 2 diabetic ex-alcoholic, Group C:
Non-diabetic alcoholic and Group D: Non-diabetic non-alcoholic control.
Following observations were obtained from the study.
Table 1: Comparison of clinical parameters among groups
|
Parameter
|
Mean
SD
|
Gr. A
(n=25)
|
Gr. B
(n=25)
|
Gr. C
(n=25)
|
Gr.D
(n=25)
|
p value for comparison
|
|
A/B
|
A/C
|
A/D
|
B/C
|
B/D
|
C/D
|
|
Age
Years
|
Mean
|
51.7
|
50.4
|
46.8
|
54.9
|
0.33
|
0.03
|
0.18
|
0.10
|
0.10
|
0.008
|
|
SD
|
10.2
|
10.8
|
8.2
|
13.9
|
|
Duration of diabetes (month)
|
Mean
|
37.8
|
83.9
|
NA
|
NA
|
0.05
|
NA
|
NA
|
NA
|
NA
|
NA
|
|
SD
|
64.4
|
123.8
|
NA
|
NA
|
|
Duration of Alcohol (year)
|
Mean
|
23.12
|
18.72
|
18.80
|
NA
|
0.03
|
0.05
|
NA
|
0.49
|
NA
|
NA
|
|
SD
|
7.46
|
8.90
|
10.63
|
NA
|
|
Smoking
(pack-years)
|
Mean
|
20.3
|
12.1
|
11.3
|
7.4
|
0.14
|
0.12
|
0.05
|
0.42
|
0.13
|
0.19
|
|
SD
|
35.3
|
12.0
|
13.7
|
16.8
|
|
Height
cm
|
Mean
|
166.1
|
163.6
|
167.2
|
165.1
|
0.18
|
0.30
|
0.32
|
0.10
|
0.28
|
0.17
|
|
SD
|
7.2
|
10.9
|
8.1
|
6.8
|
|
Weight
Kg
|
Mean
|
60.56
|
62.08
|
57.92
|
60.96
|
0.33
|
0.23
|
0.45
|
0.11
|
0.36
|
0.18
|
|
SD
|
12.46
|
11.54
|
12.21
|
11.16
|
|
BMI
Kg/m2
|
Mean
|
22.10
|
23.38
|
20.76
|
22.26
|
0.19
|
0.17
|
0.45
|
0.03
|
0.18
|
0.09
|
|
SD
|
5.33
|
5.06
|
4.40
|
3.28
|
|
Waist Circum.
|
Mean
|
86.88
|
88.12
|
81.24
|
83.96
|
0.37
|
.048
|
0.20
|
0.01
|
0.08
|
0.14
|
|
SD
|
14.55
|
10.84
|
7.70
|
9.79
|
|
WHR
|
Mean
|
0.93
|
0.95
|
0.92
|
0.92
|
0.18
|
0.33
|
0.26
|
0.049
|
0.03
|
0.39
|
|
SD
|
0.10
|
0.07
|
0.06
|
0.07
|
|
Systolic BP
mm Hg
|
Mean
|
128.2
|
136.7
|
130.3
|
122.6
|
0.07
|
0.37
|
0.17
|
0.17
|
0.01
|
0.12
|
|
SD
|
19.0
|
21.2
|
25.3
|
21.6
|
|
Diastolic BP
mm Hg
|
Mean
|
83.4
|
82.1
|
80.0
|
77.5
|
0.38
|
0.23
|
0.06
|
0.30
|
0.09
|
0.27
|
|
SD
|
13.5
|
11.3
|
16.7
|
12.0
|
* NA not applicable, significant p
values are marked in bold letters.
The table above depicts comparison of clinical and anthropometric
characteristics of the study groups. It is seen that the non-diabetic alcoholic
group (Group C) were younger than other groups. They also had a significantly
lower Body Mass Index (BMI), Waist circumference and Waist-Hip Ratio (WHR). The
diabetics who were continuing to imbibe alcohol (Group A) had a shorter
duration of diabetes (37.8 ± 64.4 months) compared to ex-alcoholic diabetics
(Group B), who had a mean duration of 83.9 ± 123.8 months, but the difference
did not reach statistical significance. As expected, alcoholics had higher
exposure to smoking but this was not a statistically significant confounding
factor. Patients belonging to group B had a significantly higher systolic blood
pressure [136.7 ± 21.2 compared to 122.6 ± 21.6, p value =0.01] compared to the
control group (Group D) as well as a higher WHR.
Table 2: Comparison of biochemical parameters in study groups
|
Parameter
|
Mean
SD
|
Gr. A
|
Gr. B
|
Gr. C
|
Gr. D
|
p value for comparison
|
|
A/B
|
A/C
|
A/D
|
B/C
|
B/D
|
C/D
|
|
Plasma Glucose
Fasting
|
Mean
|
154.7
|
154.8
|
85.5
|
78.1
|
0.50
|
<0.01
|
<0.01
|
<0.01
|
<0.01
|
0.06
|
|
SD
|
85.8
|
81.9
|
17.8
|
14.4
|
|
Plasma Glucose
PostPrandial
|
Mean
|
215.6
|
183.6
|
104.8
|
96.8
|
0.08
|
<0.01
|
<0.01
|
<0.01
|
<0.01
|
0.18
|
|
SD
|
79.5
|
77.0
|
34.4
|
24.9
|
|
Serum Urea
(mg/dl)
|
Mean
|
31.3
|
38.2
|
34.3
|
34.3
|
0.12
|
0.19
|
0.30
|
0.26
|
0.30
|
0.50
|
|
SD
|
11.1
|
27.0
|
13.3
|
25.0
|
|
Serum creatinine (mg/dl)
|
Mean
|
1.06
|
1.39
|
1.12
|
1.12
|
0.15
|
0.35
|
0.35
|
0.20
|
0.21
|
0.48
|
|
SD
|
0.55
|
1.49
|
0.57
|
0.66
|
|
SGOT
IU/L
|
Mean
|
26.8
|
25.8
|
39.0
|
25.0
|
0.45
|
0.13
|
0.42
|
0.06
|
0.45
|
0.07
|
|
SD
|
38.8
|
16.6
|
38.3
|
26.5
|
|
SGPT
IU/L
|
Mean
|
28.6
|
24.9
|
35.0
|
28.0
|
0.27
|
0.21
|
0.47
|
0.07
|
0.30
|
0.19
|
|
SD
|
26.2
|
14.0
|
30.9
|
26.0
|
|
Total Cholesterol
(mg/dl)
|
Mean
|
184.8
|
188.5
|
169.7
|
160.1
|
0.39
|
0.12
|
0.03
|
0.049
|
0.01
|
0.19
|
|
SD
|
51.4
|
40.5
|
38.3
|
37.9
|
|
Triglyceride
(mg/dl)
|
Mean
|
176.7
|
184.6
|
140.2
|
138.6
|
0.32
|
<0.01
|
<0.01
|
<0.01
|
<0.01
|
0.41
|
|
SD
|
55.8
|
62.6
|
24.5
|
27.0
|
* significant p values are marked in bold letters
This table compares the biochemical parameters in the four
study groups. Though the diabetics had a significantly higher fasting as well
as post‑prandial plasma glucose level than the non diabetics, there was
no significant difference in plasma glucose levels of the two non-diabetic
groups. All the four groups had similar urea, creatinine and aminotransferase
levels indicating difference in renal or hepatic function did not confound the
comparison of results. It is also seen that diabetes was associated with
significant hypertriglyceridemia as well as hypercholesterolemia but alcohol
intake in non-diabetics was not associated with lipid abnormality.
Table 3: Comparison of various measures of insulin
sensitivity/resistance and beta cell function among study group
|
Parameter
|
Mean
SD
|
Gr. A
|
Gr. B
|
Gr. C
|
Gr. D
|
p value for comparison
|
|
A/B
|
A/C
|
A/D
|
B/C
|
B/D
|
C/D
|
|
Serum Insulin
|
Mean
|
52.54
|
39.59
|
22.09
|
17.91
|
0.29
|
0.09
|
0.07
|
0.02
|
0.004
|
0.13
|
|
SD
|
112.79
|
36.53
|
16.25
|
8.91
|
|
QUICKI
|
Mean
|
0.296
|
0.281
|
0.321
|
0.329
|
0.06
|
0.02
|
0.002
|
0.0001
|
<0.0001
|
0.22
|
|
SD
|
0.042
|
0.030
|
0.040
|
0.033
|
|
HOMA-IR
|
Mean
|
23.2
|
14.1
|
4.7
|
3.5
|
0.22
|
0.06
|
0.047
|
0.001
|
0.0003
|
0.07
|
|
SD
|
56.3
|
13.6
|
3.3
|
2.0
|
|
HOMA-BCF (%)
|
Mean
|
611
|
586
|
964
|
1528
|
0.48
|
0.23
|
0.09
|
0.23
|
0.09
|
0.19
|
|
SD
|
1859
|
2038
|
1542
|
2760
|
The table above compares fasting insulin, Quantative Insulin
Sensitivity Check Index [QUICKI], Insulin Resistance by Homeostasis Model
assessment [HOMA-IR], and β-cell function as percentage normal calculated
by Homeostasis model assessment [HOMA-BCF] amongst the study groups. We found
that QUICKI was lower in diabetics who continued to take alcohol (Group A) and
they had a higher serum insulin level comparable to diabeic ex‑alcoholics
(Group B). The mean insulin resistance (HOMA-IR) in Group A was 23.2 ± 56.3,
which was higher than that of Group B and Group C ( 14.1 ± 15.6 and 4.7 ± 3.3)
but he difference did not attain statistical significance due to excessive
variance in the former group. HOMA-BCF was comparable in all 4 groups.
Table 4: Correlation
between Insulin Resitance and clinical parameters
|
Parameters
|
|
Relation with QUICKI
|
Relation with HOMA-IR
|
|
|
n
|
r
|
t
|
p
|
r
|
t
|
p
|
|
Age
|
100
|
-0.169
|
1.693
|
0.047
|
0.220
|
2.228
|
0.014
|
|
Duration of diabetes (A
& B)
|
50
|
-0.079
|
0.546
|
0.294
|
0.024
|
0.169
|
0.433
|
|
Duration of Alcohol
Intake
( Gr A,B,C)
|
75
|
-0.273
|
2.423
|
0.009
|
0.248
|
2.183
|
0.016
|
|
Amount of Alcohol (Gr
A,B,C)
|
75
|
0.096
|
0.827
|
0.205
|
-0.146
|
1.260
|
0.106
|
|
Discontinuation of
Alcohol (Gr B)
|
25
|
0.467
|
2.530
|
0.009
|
-0.128
|
0.620
|
0.271
|
|
Height
|
100
|
0.079
|
0.780
|
0.219
|
-0.059
|
0.581
|
0.281
|
|
Weight
|
100
|
-0.065
|
0.644
|
0.261
|
0.111
|
1.103
|
0.136
|
|
Body Mass Index
|
100
|
-0.116
|
1.151
|
0.126
|
0.138
|
1.381
|
0.085
|
|
Waist Circumference
|
100
|
-0.311
|
3.236
|
0.001
|
0.305
|
3.172
|
0.001
|
|
Waist Hip Ratio
|
100
|
-0.344
|
3.633
|
<0.001
|
0.105
|
1.043
|
0.150
|
|
Systolic Blood Pressure
|
100
|
-0.148
|
1.485
|
0.070
|
0.077
|
0.761
|
0.224
|
|
Diastolic Blood
Pressure
|
100
|
-0.128
|
1.280
|
0.102
|
0.095
|
0.941
|
0.175
|
 r = Correlation
co-efficient, n= number of subjects,
The table above shows correlation between various clinical
parameters and measures of insulin sensitivity and resistance as determined by
univariate regression analysis. Though QUICKI was inversely correlated to age,
HOMA-IR did not show such correlation. In alcoholics, there was significant
correlation of QUICKI and HOMA-IR with duration of alcohol intake but not with
amount of alcohol consumed. In diabetics who have given up alcohol, insulin
sensitivity (as measured by QUICKI) directly correlated with period of
discontinuation of alcohol.
Height, weight and BMI did not predict insulin resistance. On
the other hand, higher waist circumference and Waist-Hip Ratio was associated
with insulin resistance. Neither systolic nor diastolic blood pressure had
significant correlation with measure of insulin sensitivity.
Figure1:
Correlation between WHR and QUICKI
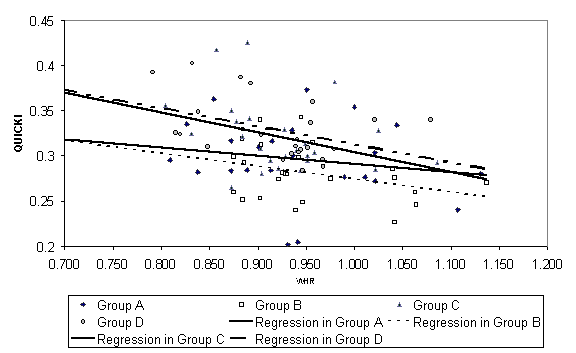
The figure above shows regression curves of Waist-Hip Ratio and
QUICKI in the four groups of patients in the study. It is seen that QUICKI steadily
decreases with rise of WHR. The correlation is more significant in non‑diabetics
(Groups C and D) as evident by steeper curve in those groups.
Figure 2: Correlation between QUICKI and duration of alcohol
intake
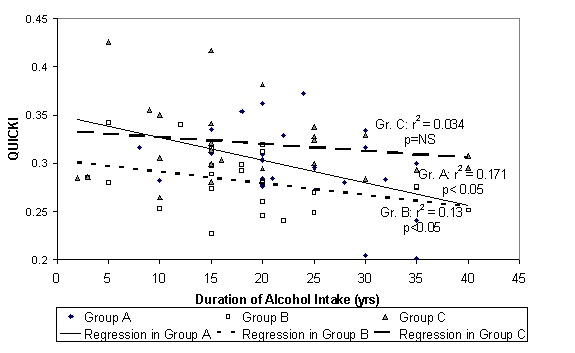
This figure shows regression curves of relation between
duration of diabetes with insulin sensitivity (QUICKI). There is definite fall
in QUICKI values indicating decline in insulin sensitivity in diabetics with
longer duration of alcohol intake. The change was most prominent in Group A,
diabetics who continued to drink (r2 =0.171, p=0.02). But in
non-diabetic alcoholics (Group C), there was no correlation between duration of
alcohol intake and QUICKI (r2 = 0.034, p not significant).
Figure 3: Correlation between amount of alcohol intake and
insulin resistance

The figure above depicts the relationship between amount of
alcohol consumption per week and insulin resistance measured by HOMA model. Due
to wide range of HOMA-IR values a logarithmic scale was taken. There was no
significant effect of amount of alcohol consumed on insulin resistance, both in
diabetics and the non-diabetic alcoholic group although there was a downward
trend, which was non-significant statistically.
Figure 4: Effect of discontinuation of alcohol intake in diabetics
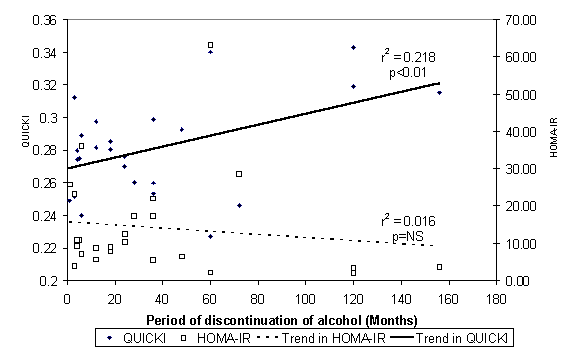
The figure above illustrates the effect of discontinuation of
alcohol in diabetics. There is an upward trend in insulin sensitivity as
measured by QUICKI with longer period of abstinence from alcohol (r2
=0.218, p<0.01).  HOMA-IR did not show any significant correlation
Table 5: Correlation between Insulin
Resistance and
Biochemical parameters
|
Parameters
|
Relation with QUICKI
|
Relation with HOMA-IR
|
|
|
r
|
t
|
p
|
r
|
t
|
p
|
|
Glucose (F)
|
-0.411
|
4.462
|
<0.001
|
0.305
|
3.168
|
0.001
|
|
Glucose
PP
|
-0.361
|
3.837
|
<0.001
|
0.368
|
3.920
|
<0.001
|
|
Urea
|
-0.193
|
1.949
|
0.027
|
-0.016
|
0.163
|
0.436
|
|
Creatinine
|
-0.166
|
1.662
|
0.050
|
0.011
|
0.107
|
0.457
|
|
SGOT
|
-0.114
|
1.136
|
0.129
|
0.023
|
0.229
|
0.409
|
|
SGPT
|
-0.190
|
1.913
|
0.029
|
0.273
|
2.808
|
0.003
|
|
Cholesterol
|
-0.214
|
2.167
|
0.016
|
0.253
|
2.584
|
0.006
|
|
Triglyceride
|
-0.392
|
4.212
|
<0.001
|
0.325
|
3.407
|
<0.001
|
Â
The table above shows the relation between biochemical marker
and insulin resistance. We can see that both fasting and post-prandial plasma
glucose levels highly correlated with insulin resistance (p< 0.001). Among
the aminotranferases, alanine aminotransferase (SGPT) was directly correlated
to insulin resitance but aspartate aminotransferase (SGOT) did not show such
correlation. Hypercholesterolaemia and hypertriglyceridemia are associated with
rise in HOMA-IR and fall in QUICKI suggesting that they are good marker for
insulin resistance.
Table 6: Comparison of various indices related
to insulin resistance in non‑diabetics according to amount of alcohol
intake
|
Amount of weekly alcohol
consumption
(g/wk)
|
|
Insulin
|
QUICKI
|
HOMA‑IR
|
HOMA‑BCF
|
|
n
|
Mean
|
SD
|
Mean
|
SD
|
Mean
|
SD
|
Mean
|
SD
|
|
0
|
25
|
17.9
|
8.9
|
0.329
|
0.033
|
3.5
|
2.0
|
1528
|
2760
|
|
1-500
|
8
|
30.9
|
21.9
|
0.307
|
0.034
|
6.0
|
4.1
|
1027
|
782
|
|
501-1000
|
9
|
20.8
|
12.6
|
0.321
|
0.041
|
4.5
|
2.8
|
1419
|
2427
|
|
1001-1500
|
5
|
16.8
|
10.6
|
0.331
|
0.052
|
4.0
|
2.9
|
462
|
448
|
|
>1500
|
2
|
8.7
|
0.14
|
0.347
|
0.012
|
1.9
|
0.5
|
180
|
153
|
As shown in the table above, non‑alcoholics had a lower
insulin level of 17.9 ± 8.9 µu/ml compared to persons consuming up to 500 gm
and 500 to 1000 gm of ethyl alcohol per week (insulin levels 30.9 ± 21.9 µu/ml
and 20.8 ± 12.6 µu/ml, respectively). On the other hand, persons consuming 1000
gm or more alcohol per week were having insulin level lower than non-alcoholic
population. This fall in insulin level was associated with a rise in QUICKI and
fall in HOMA-IR. An interesting observation obtained is that persons consuming
a heavy amount of alcohol were having β‑cell function (as determined
by HOMA equation) below that of normal persons.
However after adjusting for age, BMI and WHR by doing a
multivariate regression analysis, the correlation between amount of alcohol
consumed and insulin sensitivity becomes non‑significant
statistically(t=0.20, p=NS).
Â
Discussion
Discussion
The incidence of type 2 diabetes and its complications has
reached an alarming proportion in recent years. With the projected further rise
in the disease worldwide, particularly in India, it has become necessary to elucidate
factors which affect and modify the aetiopathogenesis of the disease, namely
insulin resistance and β‑cell dysfunction. Proper delineation of
effects of various dietary and lifestyle factors on progression of diabetes
will not only lead to better patient care in form of proper advice based on
evidence, but also help in forming a strategy in the primordial prevention of
the disease and its risk factors in community.
There has been a significant rise of alcohol consumption due to
increased urbanisation and easy availability of alcohol in India in recent
times. Quite a few studies have been done in Western as well as Far Eastern
nations which evaluated effect of regular alcohol consumption on insulin levels
and progression of diabetes both in diabetics 21,42,43 and non‑diabetics 20,22,46,47. Hence
this study was planned to assess the effect of regular alcohol intake in a
small population sample of patients attending RNT Medical College and Hospitals
consisting of both diabetics and non-diabetics who differ in their alcohol
intake. We found no study which reported the effect of alcohol in subjects of
Indian sub-continent. As this population group vary considerably, as per the
risk factors of diabetes is concerned, from occidental populace13,14 and most of the subjects
here consume country liquor of questionable quality19, the effect may
be different from what was obtained in Western literature.
A total of one hundred subjects were included in this study
with twenty five patients in each of the following groups: Group A- type
2 diabetic alcoholics; Group B – type 2 diabetic ex-alcoholics; Group
C- non‑diabetic alcoholics; and Group D- non-diabetic non-alcoholics.Â
The mean (± SD) ages of the four groups were 51.7 ± 10.2, 50.4 ± 10.8, 46.8 ±
8.2 and 54.9 ± 13.9 years, indicating that non‑diabetic alcoholics were
younger. This is due to random enrolment in the study and age standardisation
was done by multivariate regression analysis.
Though smoking was more commonly associated with alcohol
intake, the difference was not significant enough to confound the results.
On comparing the study groups, we found that diabetics had
higher Body Mass Index (BMI), Waist Circumference and Waist‑Hip Ratio
(WHR) index. These findings are in consonance with that of various previous
investigators who found that anthropometric measurements are often high in
diabetics or persons with impaired glucose tolerance29,31. Non‑diabetics
who are alcoholics (Group C) had a still lower WHR than non‑diabetic non‑alcoholics.
This may be due to (i) their lower age or (ii) poor nutritional intake
associated with alcoholism.
Ex-alcoholic diabetics had a significantly higher systolic
blood pressure compared to non-alcoholic non-diabetic controls. The frequent
co-existence of hypertension and diabetes has been reported in epidemiological
studies and is explained by presence of hyperinsulinaemia in these conditions12,59.
Diabetics were having significantly elevated plasma glucose
levels compared to non‑diabetics. Though there was no difference in
fasting glucose levels, the post-prandial levels were higher in alcoholics than
ex-alcoholics. Though acute alcohol is known to cause hypoglycemia in normal
persons by inhibiting gluconeogenesis, similar effect has not been observed in
diabetics38.
However it will be premature to comment on effect of ethanol on long-term
glycaemic control based on plasma glucose levels alone in absence of data about
glycosylated haemoglobin levels.  Even in non-diabetics, regular alcohol
consumption did not cause significant effect on plasma glucose level compared
to non-alcoholics, which is comparable to result obtained in the prospective
study by Davies MJ et al. 47. In the long term prospective studies
like Osaka study20,
alcoholics are found to be having less chance of developing diabetes unless
they are lean.
Serum cholesterol and triglyceride levels were significantly
higher in diabetic population. This may be due to higher level of serum insulin
in them. The association between hyperinsulinaemia and hypercholesterolaemia/ hypertriglyceridaemia
has also been previously documented in CARDIA Study32. However, no
significant difference was obtained in cholesterol and triglyceride levels of
diabetics differing on alcohol intake (Group A and B). This finding is in
similar to that obtained by Bertello et al. 49. Moreover
non-diabetic alcoholics and non‑diabetic non‑alcoholics did not
differ significantly in their cholesterol and triglyceride levels. This
observations was similar to that of ‘Bruneck Study’22 but differs from
that obtained by Davies et al. in their prospective study 47.
The hepatic enzymes [SGOT(AST) and SGPT(ALT)] did not differ in
alcoholics and non-alcoholics. On the other hand Kornhuber H et al 48 found that
elevated levels of these enzymes are associated with alcohol induced liver
damage. The difference may be due to differences in assay procedure or
confounding presence of other hepatotoxic element in non‑alcoholic subjects
of our study.
Various studies previously used different measures of insulin
sensitivity in measuring the effects alcohol, thereby their direct comparison
is not possible. In our study we had selected four well-established measures of
insulin sensitivity and resistance, namely fasting insulin (FI), Quantitative
Insulin Sensitivity Check Index (QUICKI), Insulin Resistance by Homeostasis
Model Assessment (HOMA-IR) and β-cell function by HOMA (HOMA-BCF). We
found that diabetics had a significantly higher serum insulin and HOMA-IR and
lower QUICKI values compared to non-diabetics. The difference became more
significant in QUICKI values due to its less variability. However alcohol
consumption did not cause significant alteration of the parameters in
non-diabetics. The fact that HOMA‑BCF was similar in all four groups
probably indicate that the pancreatic β -cells are probably not directly
affected by alcohol consumption.
While correlating various clinical parameters by univariate
analysis, we found that QUICKI is significantly and inversely correlated with
duration of alcohol intake, indicating prolonged alcohol intake decreases
insulin sensitivity. On subgroup analysis, the fall of insulin sensitivity was
most prominent in diabetic alcoholic group, whereas in non-diabetic alcoholic
the correlation was statistically non-significant. This indicates that deleterious
effects of alcohol in diabetics increase with prolonged exposure.
Amount of alcohol intake did not have any statistically
significant effect on insulin sensitivity though a downward trend in HOMA-IR
was obtained with increasing alcohol consumption. Previous studies20, 22, 46 have
shown a significant fall in insulin concentration as well as insulin resistance
with moderate amount of alcohol consumption. The difference may be due to inherent
folly of the questionnaire method adopted in determining alcohol consumption in
this study, difference in the quality of alcohol consumed and its contaminants
or due to different genetic susceptibility of our study population.
Furthermore, there was a downward trend, albeit non‑significant, in
insulin resistance with increasing amount of alcohol consumption in all three
groups. When only non‑diabetic subgroups were studied, it was found that
insulin level, insulin resistance and β‑cell function falls and
QUICKI values rise with increasing amount of alcohol consumption. This finding
was also obtained by previous investigators. However, these relations became
non-significant when other variables like age, BMI, WHR were matched by
multivariate regression analysis making us to echo the observation of Bell et
al 44that
improved insulin sensitivity associated with moderate alcohol consumption might
be a function solely of a more favourable adiposity profile. However, the
factual history, as per as exact amount of spirit consumed by an individual, is
very difficult to assess on account of an inherent personality profile of a
person consuming alcohol for a long time. All previous study such as ours have
been population based but they point in a direction which should be worked upon
using as many subjects as possible who are quantitatively documented to be
consuming alcohol such as bar-workers, people visiting pubs.
In the patients included in our study, insulin sensitivity was
inversely related to Waist Hip Ratio whereas it was not related at all to BMI.
This peculiar situation is specifically described7,60 in Asian
Indians who have a higher WHR despite having a low BMI associated with insulin
resistance.
Surprisingly, markers of insulin resistance were not correlated
with systolic and diastolic blood pressure in the study subjects. In there
population based study in New Zealand, McAuley et al 61 also did not
found any correlation between Insulin sensitivity determined by Insulin Clamp
and hypertension. On the other hand, taking fasting and postprandial insulin
levels as markers for insulin resistance, Kothari K et al 62 found increased
insulin levels in hypertensives. The cross‑sectional nature of the study
is inadequate to delineate the complex relationship between insulin resistance
syndrome and hypertension.
Among the biochemical parameters, blood glucose (fasting and
post‑prandial), SGPT, total cholesterol and triglyceride were inversely
related to QUICKI and directly related to HOMA-IR. Various previous studies63,64 have also
confirmed usefulness of these markers of insulin resistance.
When the effect of discontinuation of alcohol on insulin
sensitivity was evaluated in the diabetic ex-alcoholic group, it was found that
there is a rising trend in QUICKI with prolonged period of abstinence. This
correlation persisted even after adjusting for age, anthropometric parameters
and amount of alcohol consumed. This may indeed indicate the beneficial effect
of stoppage of alcohol consumption on improving insulin sensitivity. A
prospective long-term study is warranted to explore this association.
An attempt has been made through this pilot study to find out a
possible relation between alcohol consumption and diabetes in India as both
diabetes and alcohol consumption are on the rise. Although clear-cut scientific
inference ccould not be drawn by this small study on such a sensitive matter unless
a larger study carried on faithful alcoholics, where exact alcohol consumption
can be quantified, is performed, this study is a pointer to the fact that
chronic alcohol consumption in so called moderate amount does lead to lead to
insulin resistance and glucose intolerance. Much has been commented upon the
effects of abnormal waist-hip ration on glucose metabolism. Chronic alcohol
consumption in many patients has cased truncal obesity with increased WHR. In
our opinion this observation is significant in Indian context, whereas sporadic
reports from the West indicated that moderate alcohol consumption in not harmful
in diabetics. In our situation, where alcohol produced is of inferior quality
along with other nutritional drawbacks, alcohol consumption should be
discouraged.
Â
Summary and Conclusions
Summary and Conclusions
This study was conducted in admitted and out‑patients of Maharana
Bhupal Government Hospital. The possible effects of prolonged consumption of
ethyl alcohol on insulin resistance on diabetic and non‑diabetic
individuals were determined by measuring various clinical and biochemical
markers and calculating various indices of insulin resistance in four groups of
twenty five subjects each: (A) diabetic alcoholics, (B) diabetic ex‑alcoholics,
(C) non‑diabetic alcoholics and (D) non-diabetic non‑alcoholics.
The following conclusions could be drawn from this study: -
a. Diabetic subjects (groups A and B) in this study in general had higher
Waist-Hip Ratio (WHR), plasma glucose, total cholesterol, triglyceride and
serum insulin levels than the non‑diabetic subjects (groups C and D).
b. Alcohol consumption did not significantly alter anthropometric
variables, blood pressure, glycaemia, liver enzymes or triglyceride and
cholesterol levels in both diabetics and non‑diabetics.
c. Quantitative insulin sensitivity check index (QUICKI) was inversely and
Insulin resistance by Homeostasis Model Assessment (HOMA-IR) was directly
correlated with Waist-Hip Ratio, fasting and post-prandial plasma glucose, Alanine
Amiinotransferase (SGPT), serum fasting cholesterol and triglyceride but not
related to Body Mass Index or blood pressure.
d. Though diabetic alcoholics had higher fasting insulin and HOMA‑IR
indices than diabetic ex‑alcoholics, the difference was not significant
statistically.
e. In diabetics insulin sensitivity decreased with increasing duration of
diabetes but tended to be lower with higher consumption of alcohol.
f. In diabetic ex‑alcoholics insulin sensitivity improved
proportionate to the period of stopping alcohol.
g. In non-diabetics, increased alcohol consumption was associated with
improved insulin sensitivity but this appeared due to favourable effect of
alcohol on body composition, namely lower WHR and BMI.
This study presented an overview of literature regarding effect
of alcohol consumption in determining insulin resistance, both in diabetics and
non‑diabetics. In view of the conflicting effects obtained in various
studies, a prospective longitudinal study is required to ascertain complex
metabolic effects of ethyl alcohol on insulin resistance.
Bibliography
Bibliography
1.
American Diabetes Association
2001. Diabetes Care; 23 (supplement1). S5
2.
Amos AF, McCarty DJ, Zimmet P.
1997. The rising global burden of diabetes and its complications: Estimates and
projections for the year 2010. Diabetic Medicine; 14: S7-S85.
3.
Singh RB, Bajaj S, Niaz MA,
Rastogi SS, Moshiri M. 1998. Prevalence of type 2 diabetes mellitus and risk of
hypertension and coronary artery disease in rural and urban population with low
rates of obesity. Int J Cardiol; 66(1):65-72
4.
Ramchandran A, Snehalatha C, Latha
E, Vijay V, Viswanathan MR 1997. Rising prevalence of NIDDM in urban population
of India. Diabetologia;
40: 232-7.
5.
Seshiah V, Balaji V, Balaji M. The
rising burden of diabetes and its complications. In Panja M (ed) Medicine
Update, vol 11, Mumbai, API; 2001: 409-412
6.
Ramachandran A, Snehalatha C, Anil
Kapur, Vijay V,Mohan V, Das AK, Rao PV 2001. High prevalence of diabetes and
impaired glucose tolerance in India - National Urban Diabetes Survey (NUDS). Diabetologia. 44: 1094
-1101
7.
Ramachandran A, Snehalatha C, Shyamala
P, Vijay V. Viswanathan M. 1994 High prevalence of NIDDM and IGT in an elderly
south Indian population with low rates of obesity. Diabetes Care. 17:1190-92.
8.
Ramachandran, A., Jali, MV.,
Mohan. V., Snehalatha, C. and Viswanathan, M. 1988 High prevalence of diabetes
in an urban population in South India. BMJ. 297: 587 590
9.
Pessin J and Saltiel AR 2000. Signaling pathways in insulin action:
molecular targets of insulin resistance. J Clin Invest;106:165-169
10.
Baumann C A and. Saltiel AR 2001. Spatial compartmentalization of
signal transduction in insulin action BioEssays 23:215-222,
11.
Powers AC. Diabetes Mellitus. In: Braunwald
E et al (eds.), HarrisonÂ’s
Principles of Internal Medicine, 15th edition, New York, Mc-Graw-Hill; 2001: 2016-7.
12.
DeFronzo RA, Ferrannini E. Insulin
resistance. A multifaceted syndrome responsible for NIDDM, obesity,
hypertension, dyslipidemia, and atherosclerotic cardiovascular disease.
Diabetes Care 1991 Mar;14(3):173-194
13.
UK Prospective Diabetes
Study Group: UK Prospective
Diabetes Study. XII: Differences between Asian, Afro-Caribbean and white
Caucasian type 2 diabetic patients at diagnosis of diabetes. Diabet Med 1994;
11(7): 670-7.
14.
Chandalia M, Abate N, Garg A,
Stray-Gundersen J, Grundy SM. 1999. Relationship between generalized and upper
body obesity to insulin resistance in Asian Indian men. J Clin Endocrinol Metab
;84(7):2329-35
15.
Schuckit MA. Alcohol and
Alcoholism. In: Braunwald E et al (eds.) HarrisonÂ’s Principles of Internal Medicine, 15th edition, New York, McGraw-Hill; 2001: 2561-3.
16.
Channabasavanna SM. Substance
abuse. In: Sainani GS (ed.) API Textbook of Medicine, 6th edition, Mumbai, API,
1999: 643-4.
17.
Sri EV, Raguram R, Srivastava M,
Alcohol problems in a general hospital--a prevalence study. 1997. J Indian Med
Assoc; 95(9): 505-506.
18.
Walia M, Agarwal AK, Shah P, Yadav
R, Singh CP, Yadav P. 1999. Prevalence of coronary risk factors in non-insulin
dependent (type 2) diabetics. J Assoc Physicians India Nov; 47(11): 1051-5.
19.
Mohan D, Sundaram KR, Advani GB,
Sharma HK, Bajaj JS. 1984. Alcohol abuse in a rural community in India. Part II: characteristics of alcohol
users Drug Alcohol Depend;14(2):121-8
20.
Tsumura K, Hayashi T, Suematsu C,
Endo G, Fujii S, Okada K. Daily alcohol consumption and the risk of type 2
diabetes in Japanese men: the Osaka Health Survey. Diabetes Care 1999 Sep; 22(9): 1432-7.
21.
Carlsson S, Hammar N, Efendic S, Persson
PG, Ostenson CG, Grill V Alcohol consumption, Type 2 diabetes mellitus and
impaired glucose tolerance in middle-aged Swedish men. Diabet Med 2000 Nov;
17(11): 776-81.
22.
Kiechl S, Willeit J, Poewe W, et
al. 1996 Insulin sensitivity and regular alcohol consumption: large,
prospective, cross sectional population study (Bruneck study). Br Med J
313:1040–1044
23.
Snehalatha C, Ramachandran A, Satyavani
K, Vijay V, Haffner SM. Specific insulin and pro-insulin concentrations in
non-diabetic South Indians. Metabolism 1998 Feb; 47(2): 230-233.
24.
Kissebah AH 1997 Central obesity:
measurement and metabolic effects. Diabetes Rev 5:8–20
25.
Pouliot M-C, Desprè³ J-P, Lemieux
S, Moorjani S, Bouchard C, Tremblay A, Nadeau A, Lupien PJ 1994 Waist
circumference and abdominal sagittal diameter: best simple anthropometric
indexes of abdominal visceral adipose tissue accumulation and related
cardiovascular risk in men and women. Am J Cardiol 73:460–468
26.
Bray G, Gray DS 1988 Treatment of
obesity: an overview. Diabetes Metab Rev 4:653–679
27.
Ashwell M, Cole TJ, Dixon AK 1985 Obesity: new insight into the anthropometric classification of
fat distribution shown by computed tomography. Br Med J (Clin Res Ed)
290:1692–1694
28.
Bernardo Lé¯ Wajchenberg
Subcutaneous and Visceral Adipose Tissue: Their Relation to the Metabolic
Syndrome. Endocrine Reviews 21 (6): 697-738
29.
Ohlson LO, Larsson B, Sv䲤sudd K,
Welin L, Eriksson H, Wilhelmsen L, BjíºŠí¾´orp P, Tibblin G 1985 The influence of
body fat distribution on the incidence of diabetes mellitus—13.5 years of
follow-up of the participants in the study of men born in 1913. Diabetes
34:1055–1058
30.
Larsson B, Sv䲤sudd K, Welin L, Wilhelmsen
L, BjíºŠí¾´orp P, Tibblin G 1984 Abdominal adipose tissue distribution, obesity
and risk of cardiovascular disease and death: 13 year follow-up of participants
in the study of men born in 1913. Br Med J (Clin Res Ed) 288:1401–1404
31.
Tai ES, Ho SC, Fok AC, Tan CE.
Measurement of obesity by anthropometry and bioelectric impedance analysis:
correlation with fasting lipids and insulin resistance in an Asian population.
Ann Acad Med Singapore
1999; 28(3): 445-50.
32.
Manolio TA, Savage PJ, Burke GL,
Liu KA, Wagenknecht LE, Sidney S, Jacobs DR Jr, Roseman JM, Donahue RP, Oberman
A. 1990 Association of fasting insulin with blood pressure and lipids in young
adults. The CARDIA study. Arteriosclerosis May-Jun;10(3):430-6
33.
DeFronzo RA, Tobin JD, Andres R
1979. Glucose clamp technique: a method for quantifying insulin secretion and
resistance. Am J Physiol.; 237:E214–E223.
34.
Bergman RN, Prager R, Volund A, Olefsky
JMÂ 1987. Equivalence of the insulin sensitivity index in man derived by the
minimal model method and the euglycemic glucose clamp. J Clin Invest.;79:790–800.
35.
Matthews DR, Hosker JP, Rudenski
AS, Naylor BA, Treacher DF, Turner RC. 1985. Homeostasis model assessment:
insulin resistance and beta-cell function from fasting plasma glucose and
insulin concentrations in man. Diabetologia; 28(7):412-9
36.
Katz A, Nambi SS, Mather K, Baron
AD, Follmann DA, Sullivan G, Quon MJ 2000. Quantitative Insulin Sensitivity
Check Index: A Simple, Accurate Method for Assessing Insulin Sensitivity in
Humans. The Journal of Clinical Endocrinology & Metabolism.; 85(7):
2402-2410.
37.
Mather KJ, Hunt AE, Steinberg HO, Paradisi
G, Hook G, Katz A, Quon MJ., Baron AD. Repeatability Characteristics of Simple
Indices of Insulin Resistance: Implications for Research Applications. J Clin Endocrinol
Metab 2001; 86: 5457–64.
38.
Christiansen C, Thomsen C,
Rasmussen O, Hansen C, Hermansen K. The acute impact of ethanol on glucose,
insulin, triacylglycerol, and free fatty acid responses and insulin sensitivity
in type 2 diabetes. Br J Nutr 1996 Nov; 76(5): 669-75.
39.
Patel DG, Singh SP. 1979. Effect
of ethanol and its metabolite on glucose mediated insulin release from isolated
islets of rats. Metabolism; 28:85-9.
40.
Johnson KH, Bazargan M, Bing EG.
2000. Alcohol consumption and compliance among inner-city minority patients
with type 2 diabetes mellitus. Arch Fam Med; 9(10): 964-70.
41.
Bastyr EJ 3rd, Huang Y, Brunelle
RL, Vignati L, Cox DJ, Kotsanos JG 2000. Factors associated with nocturnal hypoglycaemia
among patients with type 2 diabetes new to insulin therapy: experience with
insulin lispro. Diabetes Obes Metab;2(1):39-46.
42.
Solomon CG, Hu FB, Stampfer MJ, Colditz
GA, Speizer FE, Rimm EB, Willett WC, Manson JE. Moderate alcohol consumption
and risk of coronary heart disease among women with type 2 diabetes mellitus.
Circulation 2000 Aug 1; 102(5): 494-9.
43.
Valmadrid CT, Klein R, Moss SE,
Klein BE, Cruickshanks KJ. Alcohol intake and the risk of coronary heart
disease mortality in persons with older onset diabetes mellitus. JAMA 1999;
282(3): 239-46.
44.
Bell RA, Mayer-Davis EJ, Martin
MA, D'Agostino RB Jr, Haffner SM 2000. Associations between alcohol consumption
and insulin sensitivity and cardiovascular disease risk factors: the Insulin
Resistance and Atherosclerosis Study. Diabetes Care; 23(11):1630-6.
45.
Singh SP, Kumar Y, Snyder AK, Ellyin
FE, Gilden JL. 1988 Effect of alcohol on glucose tolerance in normal and non
insulin-dependent diabetic subjects. Alcohol Clin Exp Res; 12(6):727-30.
46.
Mayer EJ, Newman B, Quesenberry CP
Jr, Friedman GD, Selby JV. 1993. Alcohol consumption and insulin
concentrations. Role of insulin in associations of alcohol intake with
high-density lipoprotein cholesterol and triglycerides.Circulation;88
(5/1):2190-7
47.
Davies MJ, Baer DJ, Judd JT,.
Brown ED, Campbell WS, Taylor PR 2002. Effects of Moderate Alcohol Intake
on Fasting Insulin and Glucose Concentrations and Insulin Sensitivity in
Postmenopausal Women. JAMA ; 287 (19):
48.
Kornhuber HH; Backhaus B; Kornhuber
AW; Kornhuber J 1990. Die Hauptursache von Diabetes (Typ II): der "normale"
Alkoholkonsum.(German) Versicherungsmedizin; 42(5): 132, 134-42.
49.
Bertello PD, Gurioli LZ, Gurioli
LR, Magro E. 1989 [Effect of chronic alcohol consumption on blood lipid levels
and angiopathy in diabetics(Italian)] Minerva Med ; 80(5): 443-6.
50.
Singh RB, Rastogi SS, Rao PV, Das
S, Madhu SV, Das AK, Sahay BK, Fuse SM, Beegom R, Sainani GS, Shah NA 1997. J Cardiovasc
Risk; 4(3): 201-8.
51.
Hrebicek J, Janout V, Malincikova
J, Horakova D, Cizek L. 2002 Detection of insulin resistance by simple
quantitative insulin sensitivity check index QUICKI for epidemiological
assessment and prevention. J Clin Endocrinol Metab Jan;87(1):144-7
52.
Trinder P. 1969. Ann. Clin. Biochem.;
6: 24.
53.
Allain CA, et al 1974. Clin.Chem.;20:470
54.
Fossati P 1982. Principles Clin. Chem.; 28: 2077-80.
55.
McGowan MW et al 1983. Clin.Chem.; 29: 538
56.
Yalow RS, Berson SA 1960. Immuno-assay of endogenous plasma insulin in
man. J Clinical Invest; 346:Â
57.
Pillai MRA, Bhandarkar SD.
Radioimmunoassay: Principles and Practice, 3rd Edition, Mumbai, BARC;
1998:8-10.
58.
Nakagawa S, Nakayama H, Sasaki T,
et al. 1973. A simple Method for the determination of serum free insulin levels
in insulin-treated patients. Diabetes; 22: 590-630.
59.
Manicardi V, Camellini
L, Bellodi G, et al.1986.Evidence for an association of high blood pressure and
hyperinsulinemia in obese man. J Clin Endocrinol Metab; 62: 1302-4.
60.
Ramachandran A, Snehalatha C, Vijay V, Viswanathan
M, Haffner SM 1997. Risk of NIDDM conferred by obesity and central adiposity in
different ethnic groups – A comparative anlysis between Asian Indians and
Mexican Americans and Whites. Diab REs Clin Pact; 36: 121-25.
61.
McAuley KA, Williams SM, Mann JI, et al 2001.
Diagnosing Insulin Resistance in the General Population. Diabetes Care; 24(3):
460-4.
62.
Kothari K, Mathur A, Yadav S1998.
Estimation of fasting and post oral glucose serum insulin levels in hypertensive
and obese subjects. J Assoc Physicians India; 46(6): 514-517.
63.
Mykkanen L, Haffner S, Ronnemaa T,
Bergman R, Laakso M1997: Low insulin sensitivity is associated with clustering
of cardiovascular disease risk factors. Am J Epidemiol; 146:315–321.
64.
Berglund L, Lithell H1996:
Prediction models for insulin resistance. Blood Press 5:274– 277.
Miscellaneous
Relationship between Alcohol Consumption and
Insulin Resistance
PROFORMA FOR STUDY
Case No#
·
Name______________________ Age _________ years                                         Â
·
Address_________________________
·
Reg No. _______________Â Â Â Â Â Â Â Â Â Ward/Bed No.
________/OPD
Classification
of case
1.
Group 1: Normal Subject                                                                         []
2.
Group 2: Type 2 elderly diabetic consuming moderate
alcohol                []
3.
Group 3: Type 2 diabetic who has given up alcohol                                 []
4.
Group 4: Alcoholic who is non-Diabetic                                                  []
history
of diabetes (Group 2 and 3)
1.
Diagnosed diabetic _____years _________months back at
____ years of age
2.
 Symptoms at presentation
a)
Asymptomatic/ Incidental diagnosis
b)
Polyphagia
c)
Polyuria
d)
Polydipsia
e)
Weakness
f)
Tingling /numbness
g)
Infections/Abscess
h) Others (description)Â _______________________________
3. History of Complications (if any)
history
of addiction
1.
No addiction              Yes []                          No
[]
2.
Alcohol                                   Yes []
                         No []                          Â
a)
Duration of intake ______________years
b)
Average weekly consumption = _______ ml of
____________liquor containing approximately ____% of alcohol = _____ g of
Alcohol
c)
Sweetened []Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Non-sweetened
d)
Social drinker/ Addict
e)
Stopped drinking _____year ____months back
3.
Smoking                                 Yes []                          No
[]
a)
Duration of smoking ________years
b)
No of Average bidis/ cigarettes per day __________
c)
Total exposure _______ pack-years (1 pack = 20 cigerettes/bidis)
d)
Stopped smoking _____years _____months back
4.
Chewing tobacoo                    Yes []                          No
[]
a)
Duration _____________years
5.
Others _________________________________________
family
history
|
History of
|
Diabetes
type 2
|
Diabetes
type 1
|
CVA
|
Hypertension
|
CAD
|
|
|
Father
|
|
|
|
|
|
|
|
Mother
|
|
|
|
|
|
|
|
Brother(s)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sister(s)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other(s)
|
|
|
|
|
|
|
medication
history
1.
Insulin                        Yes []                          No
[]
a)
Average daily dose _________ IU
b)
Type                     Regular           Lispro             NPH
              Lente Â
c)
Started _____year _____month ago ______ months after
diagnosis of DM
2.
Suphonylureas            Yes []                          No
[]
a)
Drug used (Generic) ______________
b)
Dose ______mg/day ~ Â ____% of maximum
recommended dose     [glibenclamide 20 mg, glimepiride 8 mg, glipizide 40mg, gliclazide
160mg]
c)
 Started _____year _____month ago _____ months after
diagnosis of DM
3.
Meglitinide(Repaglinide) Â Â Â Â Â Â Â Yes []Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â No
[]
a)
Dose _______mg/d
b)
Started _____year _____month ago ______ months after diagnosis
of DM
4.
Biguanide (Metformin) Â Â Â Â Â Â Â Â Â Â Yes []Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â No
[]
a)
Dose ________mg/d
b)
Started _____year _____month ago ______ months after
diagnosis of DM
5.
Acarbose                                Yes []                          No
[]
6.
Thiozolidinediones     Yes []                          No
[]
a)
Drug _________________ dose ________mg/d
b)
Started _____year _____month ago ______ months after
diagnosis of DM
7.
Others
a)
β-blockers
                       Yes []                          No []
b)
ACE inhibitors     Yes []                          No
[]
c)
Diuretics               Yes []                          No
[]
d)
Aspirin                  Yes []                          No
[]
e)
Others
Assessment of Compliance     Good []           Moderate []     Poor
[]
physical
examinationation
|
Height
|
cm
|
Weight
|
Kg
|
BMI
|
|
|
Waist Circ.
|
cm
|
Hip Circ.
|
cm
|
WHR
|
|
|
Pulse
|
/min
|
SBP
|
mm Hg
|
DBP
|
mm HG
|
Findings                                                                                                                                                                                                                                                                                                         Present                                                                                                                                                           Absent
§
Cataract                                                    []                                  []                                    Â
§
Retinopathy                                              []                                   []                                    Â
§
Skin changes                                             []                                   []
§
Xanthoma                                                 []                                   []
§
Edema                                                      []                                   []
§
Neuropathy                                              []                                   []
§
Diabetic foot                                            []                                   []
§
Nephropathy                                            []                                   []
investigations
|
TEST
|
VALUE
|
NORMAL VALUE
|
|
Venous Plasma Glucose (fasting)
|
mg/dl
|
<126 mg/dl
|
|
Venous Plasma Glucose (2hr Postprandia)
|
mg/dl
|
<200 mg/dl
|
|
Serum Urea
|
mg/dl
|
15-40 mg/dl
|
|
Serum Creatinine
|
mg/dl
|
0.7-1.5 mg/dl
|
|
Urine Sugar
|
|
Nil
|
|
Urine Albumin
|
|
Nil
|
|
Urine Ketone
|
|
Nil
|
|
Urine Microscopy
|
|
|
|
SGOT
|
IU/L
|
5-25 IU/L
|
|
SGPT
|
IU/L
|
5-25 IU/L
|
|
SPECIAL INVESTIGATIONS
|
|
|
|
Serum Insulin (Fasting)
|
mU/ml
|
5-25 mU/ml
|
|
Total Cholesterol(Fasting)
|
mg/dl
|
<200 mg/dl
|
|
Triglyceride(Fasting)
|
mg/dl
|
< 150 mg/dl
|
|
USG Abdomen
For liver and Portal vein
|
|
|
Consent Form
I consent for inclusion in this study voluntarily and collection of
blood sample and personal data for the same.
Signature of Patient
अनुमति
पत्र
मैं किये
जा रहे शोध के लिये
व्यक्तिगत जानकारी
व खुन कि जाँच की
अनुमति देता हुँ
हस्ताक्षरÂ
रोगी




