
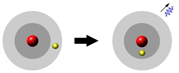
Nobody knows what an atom looks like, or even if it makes sense to suppose it has an appearance. To visualize the processes that occur at the subatomic realm, we construct models. In the planetary model - the one that most people think of when they picture the atom - the electrons orbit the nucleus like planets going around the sun. This was an early model of the atom suggested by the Danish physicist, Niels Bohr in 1913. He said that electrons are found at certain distances from the nucleus called energy levels. Levels closer to the nucleus correspond to lower energy and those further from the nucleus to higher energy. Use the following applet to answer the questions below.


QUESTIONS:
1. When hit, each bar of the xylophone
represents an incoming photon. Which bar represents
a) the shortest wavelength?
b) the highest frequency?
c) the highest energy?
2. Hit each bar of the xylophone and record what happens when the bar is hit and after you wait a bit. Do all the bars cause something to happen?
3. Hit the green bar of the xylophone and then the red bar. What happens first? Then what happens? Repeat this several times until you get a end different result after you wait a bit.
4. How do you make an electron jump up to a higher energy level?
5. What happens when an electron jumps down to a lower energy level

Read Spectral Lines, Bohr's Atom, Energy Levels, and Atomic Spectra (you can also use the "Next" button at the bottom of each of these pages).
6. Why could atomic line spectra be called the "fingerprints" of atoms?
7. How does the Bohr model of the atom explain line spectra?