Unit 3 Study Guide, Part 1
Chemical Bonding - Ionic
Targets:
E5. Describe how atoms are
joined by chemical bonding.
H9. Demonstrate an
understanding that energy can be found in chemical bonds and can be used when
it is released from those bonds.
Activity #1 Introduction to Ionic Bonding
Open Chemical
Bonding. Define the words and answer
the questions. The definitions can be
found by clicking on the word in the reading.
1)
Define:
a)
element
b)
compound
2)
There are 118 or so elements on the periodic
table. Why are there many more than 118
substances found in nature?
3)
What did the American chemist Gilbert Newton Lewis
propose in 1916 about the reason for chemical bonding?
4)
Define
a)
valence shell
b)
valence electrons
5)
Lewis determined that elements are most stable when
they contain how many electrons in their outer shell?
6)
What do elements with incomplete valence shells tend to
do?
7)
Define
a)
ion
b)
ionic bond
c)
ionic compound
8)
Watch the Flash movie showing the reaction
of sodium and chlorine.
a)
Describe the properties of :
i)
sodium:
ii)
chlorine:
b)
Drop the sodium into the chlorine gas. What happens?
c)
Magnify the reaction.
i)
Does sodium lose or gain an electron?
ii)
Does chlorine lose or gain an electron?
iii)
What is the charge of the sodium ion?
iv)
What is the charge of the chlorine ion?
v)
What holds the sodium and chlorine ions together?
d)
Click What compound is formed?.
i)
What is the common name for sodium chloride?
ii)
Describe the properties of sodium chloride.
iii)
Do compounds keep the properties of the elements that
make them up? Explain.
Activity #2 Bohr Diagram Review
Open 3.3.1a - Bohr Diagram.
Read the explanation of Bohr diagrams. In this tutorial p+ is the symbol for a proton and e is the symbol for an electron.
Remember that
- the total
number of electrons in a neutral atom is equal to the number of protons
given by the atomic number on the periodic table
- the maximum
number of electrons in the 1st energy level is 2 and in the 2nd
energy level is 8


1)
Look at a periodic table and the Bohr diagrams above.
a)
In what group are hydrogen and lithium on the periodic
table? ____ A
b)
How many valence electrons (electrons in the outer
shell) do hydrogen and lithium have? _____
Remember: For A group representative
elements, group # = # of valence e
2) Atoms
that have full valence shells are very stable (chemically inert) and do not
tend to form compounds. In what group would you find the most stable elements
on the periodic table? Why? Check your
answer here.
3)
Draw Bohr diagrams for the following noble gases. Fill in the group number and the number of
valence electrons (electrons outermost energy level). (Check your answers here.
a)
helium (He)
b)
neon (Ne)
c)
argon (Ar)
4) Why
do you think helium (with 2 valence electrons) is in the same group as the
other noble gases (with 8 valence electrons)?
Read Introduction
to Ionic Compounds and fill in the blanks.
5)
The formation of an IONIC BOND is the result of the ____________ of one or more
___________ from a ______________ onto a _______________.

___________,
with only a few electrons in the outer energy level, tend to _________electrons
most readily. The energy required to remove an electron from a neutral atom is
called the _________________ __________________.
Energy + Metal Atom ΰ Metal (+) ion + e-
________________,
which lack only one or two electrons in the outer energy level have little
tendency to lose electrons - the ionization potential would be very high.
Instead ______________ have a tendency to ____________ electrons. The
________________ ________________ is the energy given off by an atom when it
gains electrons.
Non-metal Atom + e- ΰ Non-metal (-) ion +
energy
Read
Formation of
Positive Ions.

6)
Consider the group 1A metal, potassium (K).
a)
Predict how many valence electrons potassium will have.
___
b)
Verify your answer to part a by drawing a Bohr diagram.
Check your diagram here.
c)
What is the nearest noble gas (from question #3) to
potassium?
d)
How will potassium complete its octet?
e)
What charge would a potassium ion have?
f)
Draw the Lewis symbol for a potassium ion and check here. (Note: if the charge is +1 or
1, the numeral 1 can be left out and can be written as + or )
7)
Consider the group 2A metal, calcium (Ca).
a)
Predict how many valence electrons calcium will have.
___
b)
Verify your answer to part a by drawing a Bohr diagram.
Check your diagram here.
c)
What is the nearest noble gas (from question #3) to
calcium?
d)
How will calcium complete its octet?
e)
What charge would a calcium ion have?
f)
Draw the Lewis symbol for a calcium ion and check here.
Read Formation
of Negative Ions.

8)
Consider the group 7A nonmetal, chlorine (Cl).
a)
Predict how many valence electrons chlorine will have.
___
b)
Verify your answer to part a by drawing a Bohr diagram.
Check your diagram here.
c)
What is the nearest noble gas (from question #3) to
chlorine?
d)
How will chlorine complete its octet?
e)
What charge would a chlorine ion have?
f)
Draw the Lewis symbol for a chlorine ion and check here. (Note: if the charge is +1 or
1, the numeral 1 can be left out and can be written as + or )
9)
Consider the group 5A nonmetal, nitrogen (N).
a)
Predict how many valence electrons nitrogen will have.
___
b)
Verify your answer to part a by drawing a Bohr diagram.
Check your diagram here.
c)
What is the nearest noble gas (from question #3) to
nitrogen?
d)
How will nitrogen complete its octet?
e)
What charge would a nitrogen ion have?
f)
Draw the Lewis symbol for a nitrogen ion and check here.
10) Fill in the
table. Click here to check your Lewis Symbols.
|
chem. symbol |
metal or nonmetal? |
group # |
# of valence e |
# of e
( lost
/gained) |
charge of ion |
Lewis symbol |
|
Al |
metal |
3A |
3 |
3,
lost |
+
3 |
Al
3+ |
|
I |
nonmetal |
7A |
7 |
1,
gained |
1 |
I − |
|
Li |
|
|
|
|
|
|
|
Ba |
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
P |
|
|
|
|
|
|
Activity #3 Naming Ionic Compounds
Open Ionic
Compounds Activity. An ionic compound consists of cations and anions.
1) Click
the ion of lithium (Li+) and the ion of fluorine (F).
a) What
is the name of this compound?
b) Which
ion comes first in the name, the cation or the anion?
c) What
new ending does a group 7A ion get? (what replaces the ine in fluorine?)
2) Consider
an ionic compound of sodium and bromine.
a) What
do you think the name of this compound will be?
b) What
cation ion must you choose from the list?
c) What
anion must you choose from the list?
d) Was
your answer to part a correct? If not, what is the correct answer?
3) Some
ions contain more than one element (polyatomic ions) and have special
names. What is the name of the ion
a) NH4+
?
b) SO42
?
4) Transition
(group B) metals can form ions with different charges. Lets investigate how the names of compounds
containing these ions show the charge of the metal ion.
a) What
is the name of the compound containing Fe2+ and OH ?
b) What
is the name of the compound containing Fe3+ and OH ?
c) How
does the name of the compound show which iron ion it contains?
5) Predict
the names of the following compounds and then check your answers, correcting
them if you were wrong.
|
ionic compound |
cation |
anion |
|
|
Pb2+ |
SO42 |
|
|
NH4+ |
S2 |
|
|
Fe3+ |
Cl |
6) What
ions compose the following compounds? Check your answers, correcting them if
you were wrong.
|
ionic compound |
cation |
anion |
|
iron (II)
phosphate |
|
|
|
aluminum
hydroxide |
|
|
|
barium
fluoride |
|
|
Activity #4 Formulas of Binary Ionic
Compounds
Read Predicting Formulas of
Ionic Compounds and fill in the blanks.
Problem
Predict the
formulas of the ionic compounds formed by the following elements:
·
lithium and oxygen (Li and O)
·
nickel and sulfur (Ni and S)
·
bismuth and fluorine (Bi and F)
·
magnesium and chlorine (Mg and Cl)
First, look at
the locations of the elements on the _____________ _____________. Atoms in the same column as
each other (_____________) tend to exhibit similar _____________, including the
number of _______________ the elements would need to gain or lose to resemble
the nearest _____________ ____________ atom. To determine common ionic
compounds formed by elements, keep the following in mind:
·
Group I ions (alkali metals) have _____ charges.
·
Group 2 ions (alkaline earth metals) have _____charges.
·
Group 6 ions (nonmetals) have _____ charges.
·
Group 7 ions (halides) have _____ charges.
·
There is no simple way to predict the charges of
the transition metals. Look on a table
listing charges (valences) for possible values. For introductory and general
chemistry courses, the +1, +2, and +3 charges are most often used.
When you write
the formula for an ionic compound, remember that the _________ ion is always
listed first.
Write down the
information you have for the usual charges of the atoms and _________ them to
answer the problem.
CHEMICAL
FORMULA
- _____
Li+ ions are required to balance _____ O2- ion ________________
- _____ Ni 2+ ion
is required to balance _____ S2- ion ________________
- _____ Bi3+ ion
is required to balance _____ F- ions ________________
- _____ Mg2+ ion
is required to balance _____ Cl- ions
________________
Open the flash animation, Binary Ionic
Formulas.
1.
Add one cation and one anion to each side of the balance.
2.
Add another
ion to whichever side is higher.
3.
Continue adding
one ion at a time to the higher side until the positive and negative charges
balance.
4.
Record your
results in the table below.
5.
Click new
compound to get a new problem. Do 15
total.
(Note: The names of the compounds on this simulation
use an older system that we will not be using.)
|
cation (+) |
anion () |
formula unit |
||||
|
Lewis Symbol |
# used |
total + charge |
Lewis Symbol |
# used |
total charge |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In each of the
ionic compounds above, what is the sum of the total positive and negative
charges? )____________
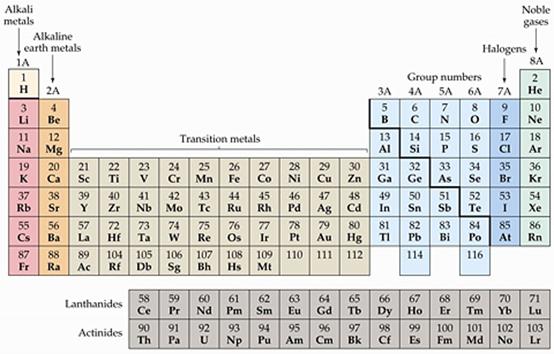
Open Nomenclature
of Binary Ionic Compounds Containing a Metal Ion With a Fixed Charge. Read
Rules for Naming Binary Ionic Compounds Containing a Metal Ion With a Fixed
Charge and do the first 10 questions, recording your answers in the table below.
Note: you may not be filling in all columns
for every question. Also note that
everyone may not have the same questions!
|
|
compound name |
formula unit |
# |
cation |
# |
anion |
|
1 |
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
4 |
|
|
|
|
|
|
|
5 |
|
|
|
|
|
|
|
6 |
|
|
|
|
|
|
|
7 |
|
|
|
|
|
|
|
8 |
|
|
|
|
|
|
|
9 |
|
|
|
|
|
|
|
10 |
|
|
|
|
|
|
Open Binary Ionic Compounds
Containing a Metal Ion With a Variable Charge. Read Rules for Naming
Binary Ionic Compounds Containing a Metal Ion With a Variable Charge and do
the first 10 questions, recording your answers in the table below.
|
|
compound name |
formula unit |
# |
cation |
# |
anion |
|
1 |
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
4 |
|
|
|
|
|
|
|
5 |
|
|
|
|
|
|
|
6 |
|
|
|
|
|
|
|
7 |
|
|
|
|
|
|
|
8 |
|
|
|
|
|
|
|
9 |
|
|
|
|
|
|
|
10 |
|
|
|
|
|
|
Activity #5 Polyatomic Ions
Open the Polyatomic
Ion Game.
A polyatomic ion is a charged particle
containing two or more covalently bonded atoms.
This game will get you familiar with some of these ions. Build the given polyatomic ion by moving the
element symbols into the boxes above. If
you need more than one atom of that element, drag more to the same box. Do the same with the charge until you have
the correct charge. There is a table of
polyatomic ions in the back of the packet to help you. Race the person at the computer next to you. The first person to 30 points wins! Have your teacher initial below.
your score ___________ teachers initials _____________
After playing
this game, would you say most polyatomic ions are negative or positive?
Open Predicting
Formulas of Compounds with Polyatomic Ions.
Read and fill in the blanks below.
Problem
Predict the formulas of these
compounds, which contain polyatomic ions:
- barium
hydroxide
- ammonium
phosphate
- potassium
sulfate
When you write the formula for an
ionic compound, remember that the _________ ion is always listed first. When
there are two or more polyatomic ions in a formula, enclose the polyatomic ion
in _________.
Write down the information you have
for the charges of the component ions and balance them to answer the problem.
CHEMICAL
FORMULA
- _____
Ba2+ ion is required to balance _____ OH- ions ________________
- _____
NH4+ ions are required to balance _____ PO43-
ion ________________
- _____
K+ ions are required to balance _____ SO42-
ion ________________
Open Ionic
Compounds Containing Polyatomic Ions. Read Rules for Naming Ionic
Compounds Containing Polyatomic Ions and do the first 20 questions, recording
your answers below.
|
|
compound name |
formula unit |
# |
cation |
# |
anion |
|
1 |
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
4 |
|
|
|
|
|
|
|
5 |
|
|
|
|
|
|
|
6 |
|
|
|
|
|
|
|
7 |
|
|
|
|
|
|
|
8 |
|
|
|
|
|
|
|
9 |
|
|
|
|
|
|
|
10 |
|
|
|
|
|
|
|
11 |
|
|
|
|
|
|
|
12 |
|
|
|
|
|
|
|
13 |
|
|
|
|
|
|
|
14 |
|
|
|
|
|
|
|
15 |
|
|
|
|
|
|
|
16 |
|
|
|
|
|
|
|
17 |
|
|
|
|
|
|
|
18 |
|
|
|
|
|
|
|
19 |
|
|
|
|
|
|
|
20 |
|
|
|
|
|
|
Activity #6
Ionic Compound Naming & Formula Review
Open the links for
Part 1 and Part 2 and complete the table.
|
# |
compound name |
formula unit |
|
1 |
|
Al2O3 |
|
2 |
|
NH4NO3 |
|
3 |
|
SrSO4 |
|
4 |
|
Ba(ClO3)2 |
|
5 |
|
Mg(OH)2 |
|
6 |
|
KHCO3 |
|
8 |
|
Hg2O |
|
10 |
|
Cu2O |
|
11 |
|
KMnO4 |
|
12 |
|
NaC2H3O2 |
|
13 |
|
Ba(ClO)2 |
|
14 |
|
CoCr2O7 |
|
15 |
|
BeS |
|
16 |
|
NaCN |
|
17 |
|
PbO2 |
|
18 |
|
NH4HSO4 |
|
1 |
qluminum oxalate |
|
|
2 |
calcium fluoride |
|
|
3 |
the Roman
numeral in ionic formulas refers to |
o the
charge on the cation o the
charge on the anion o the
number of cations o the
number of anions o none
of these |
|
4 |
potassium dihydrogen phosphate |
|
|
5 |
zinc perchlorate |
|
|
6 |
ammonium
chloride |
|
|
7 |
sodium bicarbonate |
|
|
8 |
platinum(IV)
chloride |
|
|
9 |
strontium
nitride |
|
|
10 |
potassium
dichromate |
|
|
11 |
iron(III)
oxide |
|
|
12 |
potassium
permanganate |
|
|
13 |
sodium Acetate |
|
|
14 |
cesium bromide |
|
Predicting
Monatomic Cation Charges
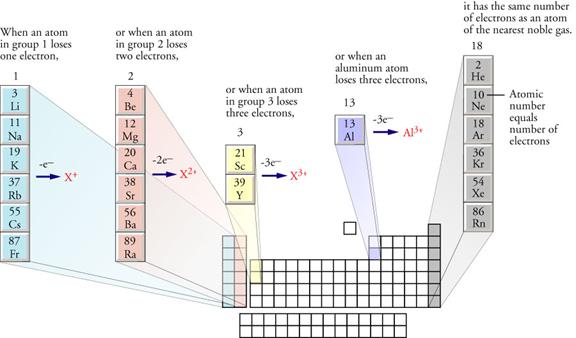
Predicting Monatomic Anion Charges

POLYATOMIC IONS
Symbol Name CH3COO1 acetate ion NH41+ ammonium ion AsO43
arsenate ion C6H5COO1
benzoate ion HCO31 bicarbonate ion BrO31 bromate ion CO32 carbonate ion ClO31 chlorate
ion ClO21 chlorite
ion CrO42 chromate
ion C6H5O73
citrate ion CN1 cyanide ion Cr2O72
dichromate ion OH1 hydroxide ion ClO1 hypochlorite ion IO31 iodate
ion PO31 phosphite
ion NO31 nitrate ion NO21 nitrite ion C2O42
oxalate
ion ClO41 perchlorate ion IO41 periodate
ion MnO41 permanganate ion PO43 phosphate ion SiO32 silicate
ion SO42 sulfate ion SO32 sulfite ion S2O32
thiosulfate ion |
MONATOMIC IONS Symbol Name Cd2+ cadmium ion Cr2+ chromium (II) ion Cr3+ chromium (III) ion Co2+ cobalt (II) ion Co3+ cobalt (III) ion Cu1+ copper (I) ion Cu2+ copper (II) ion Au1+ gold (I) ion Au3+ gold (III) ion Fe2+ iron (II) ion Fe3+ iron (III) ion Pb2+ lead (II) ion Pb4+ lead (IV) ion Pt2+ platinum (II) ion Pt4+ platinum (IV) ion Sn2+ tin (II) ion Sn4+ tin (IV) ion Ti3+ titanium (III) ion Ti4+ titanium (IV) ion W4+ tungsten (IV) ion W5+ tungsten (V) ion U3+ uranium (III) ion U4+ uranium (IV) ion U5+ uranium (V) ion U6+ uranium (VI) ion V3+ vanadium (III) ion V4+ vanadium (IV) ion V5+ vanadium (V) ion |