 Abstract/Summary
Abstract/Summary 
Caffeine is a chemical found in coffee, tea, cola and chocolate. It is produced naturally by plants. I investigated caffeine for pesticidal properties against drosophila. In my hypothesis I indicated that caffeine would be detrimental to drosophila. I based this claim on my research. Essentially, caffeine can substitute for adenosine – an inhibitory neurotransmitter. Caffeine prevents the "slow down" message of adenosine from being passed between nerve cells and substitutes its own "speed up" message. Overactive neurons have many hormonal ramifications (including the release of adrenaline.) To analyze the effect of caffeine on the flies, I created several different caffeine solutions and mixed them with the food supply. I then placed the flies in their respective containers, and observed them for fatalities, behavior, and life cycle data. The data that I found indicates that caffeine is effective as a pesticide: by day 8, all of the flies in the 10 g/L group and the 20 g/L group were dead. The caffeine postponed the life cycle of the 1 g/L group (the 20 g/L and 10 g/L group did not lay eggs). Additionally, the Time to Settle – a relative measurement of the activity of the flies – was greater for the higher concentrations of caffeine. I concluded that caffeine affected the flies as predicted. The overactive neurons contributed to greater activity in the flies and influenced the hormonal gland of the flies – this was the cause of the delay in the life cycle. The fly fatalities resulted from the failure of overactive organs – these were caused by the release of excessive adrenaline. As an extension to my project, I sprayed caffeine on the flies. The results from spraying were similar to ingestion, leading me to believe that caffeine can successfully penetrate the exoskeleton. For further research I could analyze the practicality of caffeine as a pesticide (Does it kill other harmful insects? Does it break down when in the environment?) I could also take an in depth look at the effect of caffeine on the flies (i.e. potential mutagenic effects, the response to specific stimuli, etc.)
 Literary Research
Literary Research 
Caffeine is a chemical found in coffee, tea, cola, and several other plant products. It is produced naturally by plants. Caffeine, in its various forms, is consumed by millions of people each day; however, caffeine has been shown to have some detrimental effects.
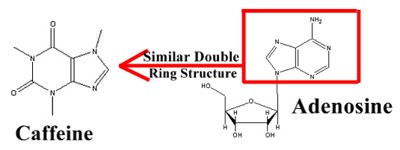
The source of caffeine's "kick" and it's dangers lie in the molecular similarity between it and adenosine. Adenosine, a primary component of ATP and DNA, fills an important role in the brain and central nervous system. Adenosine belongs to a class of chemicals known as neurotransmitters. Neurotransmitters are the communication pathway between neurons (nerve cells). Every nerve cell is composed of a central body and several long, tentacle-like structures called axons. When the nerve cell receives an impulse, the membrane of the fiber becomes depolarized, and sodium ions move across the membrane into the cell. After the membrane becomes depolarized at one point, the adjacent sections of membrane also become depolarized. Neurotransmitters like adenosine become important when the impulse reaches the end of the fiber, or synapse. A nerve impulse sets off the release of certain neurotransmitters in the synapse. The released neurotransmitters are then received by chemical-specific receptors in the adjacent synapse. These receptors only receive molecules of a certain structure.

When few nerve impulses are being communicated, the synapse releases adenosine. Adenosine is an inhibitory neurotransmitter – it tells the neurons not to fire. Additionally, adenosine stimulates the production of other inhibitory neurotransmitters. When caffeine enters an organism, it fits into the adenosine receptors - Not only does caffeine have a similar structure to adenosine, but it is also more nucleophilic and electrophilic and is accepted over adenosine by the adenosine receptors. Caffeine blocks out the "slow down" message of adenosine, while substituting it's own "speed up" message. It stimulates the release of other excitatory neurotransmitters (nor-adrenaline and dopamine) while retarding the release of inhibitory neurotransmitters (GABA and serotonin). Essentially, caffeine spurs the neurons to high levels of activity.
Additionally, caffeine acts as a phosphodiesterase inhibitor. Phosphodiesterase is an enzyme located in cells. It breaks down the cyclic nucleotides cAMP and cGMP, which "are second messengers that carry signals from the cell surface to proteins within the cell." (http://www.mblab.gla.ac.uk/tubules/map/pde.html) If these messenger molecules are not broken down, they simply continue to deliver their message, again and again, to the proteins within the cell. The result: a stimulus that continues to stimulate without cease. Cyclic nucleotides deliver excitatory messages; therefore, by allowing them to continuously deliver their message, cell activity is increased. Caffeine, which inhibits the production of phosphodiesterase, thereby leaves the cyclic nucleotides free to deliver their excitatory message again and again, and results in an increase in cell activity.
The consequences of high neuron activity are numerous. The neurons, which are linked to the organs of hormonal release, have many hormonal consequences. Primarily, there is an increase in the amount of adrenaline released. Adrenaline, a hormone that directs cells to release glucose into the blood stream, stimulates a fight of flight reaction in an organism.
Drosophila, although significantly smaller than humans, have a fairly sophisticated central nervous system and endocrine system. Their brain is connected to a thoracic ganglia (a mass of nerves) by the cervical connective. This complex nervous system means one thing: there are many nerve cells for caffeine to affect. Drosophila has an organ of hormonal release called the corpus allatum. The corpus allatum releases countless hormones, including adrenaline. These hormones have similar effects on drosophila as on humans: in his Insect Hormones, Wigglesworth notes that "adrenaline will produce pharmacological effects on the heart beat, gut movements, etc., in insects." (Wigglesworth, p. 101) Insects have many of the same neurotransmitters as humans: including GABA, dopamine (in high amounts), serotonin (5-HT), and nor-adrenaline. These chemicals perform the same functions in insects as in humans: "Dopamine induces a current in cockroaches [fires the neurons]" (Burrows, p. 185) and "catecholamines…[dopamine and nor-adrenaline] act as synaptic transmitters." (Wigglesworth, p. 101) Like humans, insects have 3 types of neurons: sensory, association, and motor. (Demerec, p. 481) The variety of neurons opens several different functions to the influence of caffeine.

A message received by the nervous system is transported through the human body in less than a second. The consequence of this rapid communication is that chemicals like caffeine influence the entire body shortly after they are introduced.
From this research, it is feasible that caffeine would have a detrimental effect on flies and other organisms. Several studies have already been conducted involving the effect of caffeine on flies, rats, etc. One group experimented with the effect of caffeine on the social investigatory behavior of the male Norway rat: "At a dosage of 20 mg/kg [body weight] or greater, caffeine counteracts a decrease in social investigation attributed to copulatory experience. At a 10 mg/kg dosage, caffeine increases social investigatory behavior prior to sexual exposure but has no comparable effect after sexual exposure…[they concluded that] acute caffeine exposure apparently interferes with access or retrieval of reference information in long-term memory." (Thor) Another group experimented with Drosophila: "The productivity of Drosophila prosaltan treated with six concentrations of caffeine (from 50 micrograms/ml [.5mg/L] to 2,500 micrograms/ml [2.5 mg/L] of culture medium) during ten generations (approximately 8 months) decreased in a dosage dependent manner in every generation, but at the end of the treatment the flies in all experiments recovered normal productivity, except for those treated with 2,500 micrograms/mL. Longevity in the tenth generation was significantly reduced in males and females only in the 2,500 micrograms/ml dosage, with males being much more affected than females. In a previous study in which the treatment was done in a single generation, productivity exhibited only a partial recovery when the treatment ceased and longevity was significantly reduced in 1, 500 micrograms/ml dosages. The hypothesis of selection occuring in ten generations leading to recovery in productivity and to a reduction in the processes which cause a decrease in longevity is being considered." (Itoyama) In another experiment, "Parameters of sexual behaviour were studied in Drosophila prosaltans treated with 2,500 micrograms/ml of caffeine per 1 ml of banana culture medium. The mating frequency and copulation duration were greater in control than in treated flies, while the pre-copulation duration was greater in treated flies than in controls." (Itoyama)
Yet the question remains: Would caffeine be an effective pesticide when sprayed on insects. Research seems to indicate that caffeine would be effective when sprayed. A sprayed pesticide, if effective, would have to also be absorbed by the exoskeleton. "The exoskeleton, integument, or body wall of an insect is a very remarkable structure which furnishes ample protection to the animal from moisture, dryness, disease organisms, certain animal parasites, shocks, temperature, and many other dangers. It is composed of relatively thin, rigid, hard, leathery tough plates joined by thick or thin elastic and often fragile tissues." (Essig) The ability to penetrate the exoskeleton is essential for a new pesticide to be effective. Unfortunately, the exoskeleton is composed of several layers, and most have some impermeability.
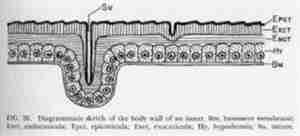
 The exoskeleton is composed of three principal layers – cuticula, hypodermis, and basement membrane. The cuticula has a stratified appearance and consists of two distinct layers – an outer cuticula, exocuticula, and an inner cuticula, endocuticula. The exocuticula is covered on the outside by a thin layer about one micron in thickness known as the epicuticula. The most characteristic substance in the exocuticula and endocuticula is chitin. This compound is a nitrogenous polysaccharide and is insoluble and resistant to the action of water, alcohol, and diluted alkalis and acids. The epicuticula is nonchitinous in nature. It is composed of substances that protect insects from excessive dessication, humidity, and disease; thus enabling them to live under a wider range of environmental conditions. The hypodermis consists primarily of a single layer of cells which secretes the cuticula. The basement membrane is a thin, noncellular membrane that forms the inner lining of the hypodermis. (Little)
The exoskeleton is composed of three principal layers – cuticula, hypodermis, and basement membrane. The cuticula has a stratified appearance and consists of two distinct layers – an outer cuticula, exocuticula, and an inner cuticula, endocuticula. The exocuticula is covered on the outside by a thin layer about one micron in thickness known as the epicuticula. The most characteristic substance in the exocuticula and endocuticula is chitin. This compound is a nitrogenous polysaccharide and is insoluble and resistant to the action of water, alcohol, and diluted alkalis and acids. The epicuticula is nonchitinous in nature. It is composed of substances that protect insects from excessive dessication, humidity, and disease; thus enabling them to live under a wider range of environmental conditions. The hypodermis consists primarily of a single layer of cells which secretes the cuticula. The basement membrane is a thin, noncellular membrane that forms the inner lining of the hypodermis. (Little)
Chitin, the substance found in the endocuticle and exocuticle, makes up "20 to 50 per cent" (Essig) of these layers. Chitin is insoluble and impermeable to water and other solutions; therefore, these two thick, chitinous layers make a formidable barrier. The epicuticula is also impermeable, though it does not contain chitin. "The exoskeleton is made of plates or sclerites which are usually hardened or sclerotized." (Little) These hard plates are another significant barrier. The soft membrane in between these plates – sutures – are a potential doorway for pesticides.
Knowledge about the structure and function of the insect exoskeleton has proven critical in developing insecticide formulations that are able to penetrate this multi-layered protective covering…Knowledge of the nervous system of insects has led to the development of several types of insecticides designed to disrupt normal nerve function. Some of these are effective simply by contacting the insect. (http://www.nyseas.cornell.edu/ent/biocontrol/info/primer.html)
Some pesticides that affect the insect nervous system are effective simply by contact. It is feasible that a caffeine solution, which affects the nervous system, will be effective on contact. Any influence that a caffeine solution would have on a insect is due to penetration of the exoskeleton: only "insects with chewing mouthparts can be selectively controlled by some insecticides that are applied directly to plant surfaces and are only effective if ingested." (http://www.nyseas.cornell.edu/ent/biocontrol/info/primer.html)
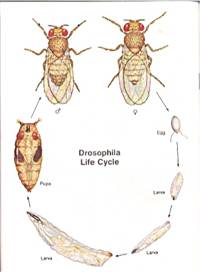
 It is also important to know a little about the life cycle of drosophila. "A fresh culture of D. melanogaster will produce new adults in two weeks; eight days in the egg and larval stages, and six days in the pupal stage. The adult fruit flies may live for several weeks…The pupa begins to darken just prior to the emergence of an adult fly." (Flagg)
It is also important to know a little about the life cycle of drosophila. "A fresh culture of D. melanogaster will produce new adults in two weeks; eight days in the egg and larval stages, and six days in the pupal stage. The adult fruit flies may live for several weeks…The pupa begins to darken just prior to the emergence of an adult fly." (Flagg)
When monitoring the flies for the negative effects of caffeine, fatalities will obviously be the primary measurement. Time to settle, however, may also have value. When Drosophila settles, it enters a sleep like state. The time that it takes to enter that state can be effectively measured.
"To facilitate the genetic study of sleep, we documented that rest behavior in Drosophila melanogaster is a sleep-like state. The animals choose a preferred location, become immobile for periods of up to 157 minutes at a particular time in the circadian day, and are relatively unresponsive to sensory stimuli. Rest is affected by both homeostatic and circadian influences: when rest is prevented, the flies increasingly tend to rest despite stimulation and then exhibit a rest rebound. Drugs acting on a mammalian adenosine receptor alter rest as they do sleep, suggesting conserved neural mechanisms." (Hendricks)
Other Pertinent Information
Caffeine:
Caffeine is a white powder; at room temperature, 1 gram is soluble in about 60 mL (Sigma)
Insects:
"Insects have three body regions: head, thorax, and abdomen. The head functions mainly for food and sensory intake and information processing…The thorax provides structural support for the legs (three pairs) and, if present, for one or two pairs of wings…The abdomen functions in digestion and reproduction.
"The internal anatomy of insects is characterized by an open circulatory system, a multitude of breathing tubes, and a three-chambered digestive system. With the exception of a heart and an aorta, there are few blood vessels; insect blood simply flows around inside the body cavity. Air enters the insect through a few openings (spiracles) in the exoskeleton, and makes its way to all areas of need by way of branching tubes, which permeate the body. The insect digestive system is long and tube-like, often divided into three sections, each with a different function. The insect nervous system transports and processes information received from the sense organs (sight smell, taste, hearing, and touch). The brain, located in the head, processes information, but some information is also processed at nerve centers elsewhere in the body." (Cornell)



