Properties of Metals
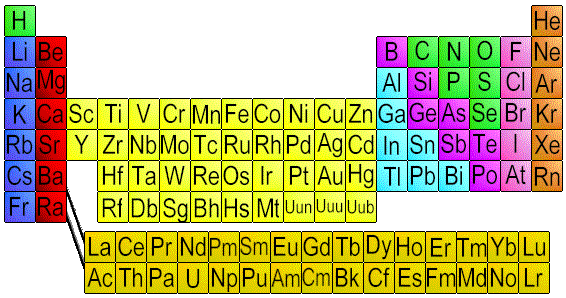
Periodic Table of Elements
The atoms of metals have either 1, 2 or 3 valence electrons. Atoms consist of a nucleus made up of positively charged protons and uncharged neutrons, as well as electrons, which are negatively charged. The electrons orbit the nucleus in shells. In the outermost electron shell (valence shell), if the number of electrons is 1, 2, or 3, then the element is a metal. Metal atoms are arranged in neat layers and are held together by the strong attraction the metal ions have for the delocalised electrons clouds. Hence, metals have high boiling and melting points. When a force is applied, the layers of atoms slide over one another readily, that is why metals can be pulled into cables (ductile) or hammered into sheets (malleable). Besides being ductile and malleable, they are good conductors of electricity, since their electron clouds are mobile. Metals generally have high densities, as their ions are packed very closely together, thus there are a large number of ions within a given volume of the metal.