Unit I The Chemistry Strand
Date: February 2008
Text book sections 2.1 & 2.2
Chemicals in Action
Chemistry is the study of matter, its properties and its changes or transformations.
A couple of demonstrations to show reactions and how torecognize if a reaction occured.
Matter is anything that has mass and takes up space. If you can put it in a bottle its matter. In contrast, you can not put a magnetic field or light in a bottle, hence these two examples are not matter. There are three states of matter which you already know; solid, liquid and gas. Each is a function of the chemical forces or bonds that hold the matter together. Strong forces of attractions would indicate a solid.
Chemicals & Chemical Change
Classifying Substances
Pure Substance: one that in which all the the particles make up the substance are the same.
Elements: are pure substances that cannot be brokes down by chemical means into simpler substances.
Elements are made up of atoms. Elements are read from the periodic chart.
Compounds: are pure substances that contain that contain two or more different elements in a fixed proportion. Are made up of compounds. Compounds are identified by chemical formulas.
Properties of Matter
All matter has both chemical and physical properties.
Physical Properties is a characteristic of a substance which can be observed using one's senses. These are qualitative observations: What does it look like, what is its colour, does it have an odour? what does its texture feel like? can the material be hammered into a sheet, etc. Properties that require measurement are called quantitative properties. Examples are: melting point, its gram solubility in water, how much heat can the material absorb (specific heat), etc.
A physical change occurs when one or more of the physical properties changes yet the material still remains that material. If you heat a solid ice cube it turns into a liquid called water, but the material is still a compound made up of hydrogen and oxygen in the ratio of 2:1.
Examples of physical changes are shown in the diagram.
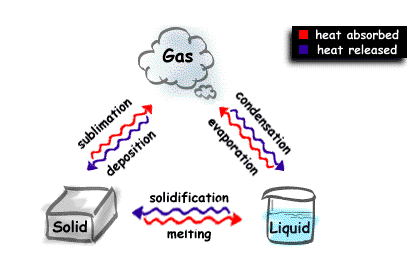
Classifying of compounds or materials
Three useful notes may be found at
- Classification of Matter

- Mixtures and Compounds

- Properties of Matter

Chemical Properties is a characteristic behavior that occurs when a substance changes into a new substance. The change itself is called a chemical change. To induce a chemical reaction either energy must be added to the material or have the material come in contact with another material or both. In a chemical reaction the starting materials are called reactants and the new materials formed are called products.
Recognizing a Chemical reaction
How do you know a chemical reaction has taken place. What is the difference between a physical change and a chemical change? First off, all chemical reactions involve the formation of a new substance. But you must be able to recognize that a new substance has been produced.
Example:
You open a can of soda and pour it into a glass: bubbles are observed.
A pinch of baking soda is added to a glass of vinegar: bubbles are observed
Which is the physical reaction and which is the chemical reaction? Answer to follow.
Symptoms of a Chemical Change or reaction
- Obvious or drastic change in appearance after the reaction.
- Bubbles of gas appear.
- A precipitate forms when two compounds are mixed.
- A colour change occurs.
- Energy such as heat or light is emitted (may also be absorbed).
- A change in smell or taste occurs (works nice with food).
- Rule of thumb: If you can't reverse the change maybe it is chemical.
Often in a chemical reaction several of the above take place simultaneously.
Eamples of the above chemical reactions may be entered in a chart to be developed and presented on the board in class.
Reactions investigated
- sugar + sulphuric acid
- copper pennies + nitric acid
- barium nitrate + sodium sulphate
- iron (III) nitrate + potassium ferrocyanide
- sugar + potassium chlorate
Practice Quiz
Attempt the following quiz and do keep score as you try the questions.
Just click on the beaker and try the quiz.
Chemicals & Safety
 Chemicals are regulated by governement regulation both from the federal and provincial governments. These regulations (laws) dictate safety labeling of compounds used in the household and in industry. The regulations are enactments of Law so as to protect the employee in the workplace from hazardous chemicals by ensuring that the worker has proper protection and training in the handling of hazardous chemicals as shown in the picture.
Chemicals are regulated by governement regulation both from the federal and provincial governments. These regulations (laws) dictate safety labeling of compounds used in the household and in industry. The regulations are enactments of Law so as to protect the employee in the workplace from hazardous chemicals by ensuring that the worker has proper protection and training in the handling of hazardous chemicals as shown in the picture.
Three such regulations are as follows
- Hazarous Houshoold Product Symbols HHPS see Page 72 Fig. 1
A brief explination with symbols may be found at

- Workplace Hazardous Materials Information System WHMIS see Page 72 Fig 2
- More avanced information is given on what are clled MSDS sheets or Material Safety Data Sheets and are provide whenever a chemical bought form a supplier.




