pH and Soil
- pH of soil determines fertility of soil
- Plants differ in their preference for soil acidity
- Sources of soil acidity include organic matter decay, naturally occurring acids, and acid precipitation
pH Affects Nutrients, Minerals and Growth
The effect of soil pH is great on the solubility of minerals or nutrients. Fourteen of the seventeen essential plant nutrients are obtained from the soil. Before a nutrient can be used by plants it must be dissolved in the soil solution. Most minerals and nutrients are more soluble or available in acid soils than in neutral or slightly alkaline soils.
Phosphorus is never readily soluble in the soil but is most available in soil with a pH range centered around 6.5. Extremely and strongly acid soils (pH 4.0 - 5.0) can have high concentrations of soluble aluminum, iron and manganese which may be toxic to the growth of some plants. A pH range of approximately 6 to 7 promotes the most ready availability of plant nutrients.
But some plants, such as azaleas, rhododendrons, blueberries, white potatoes and conifer trees, tolerate strong acid soils and grow well. Also, some plants do well only in slightly acid to moderately alkaline soils. However, a slightly alkaline (pH 7.4 - 7.8) or higher pH soil can cause a problem with the availability of iron to pin oak and a few other trees in Central New York causing chlorosis of the leaves which will put the tree under stress leading to tree decline and eventual mortality.
The soil pH can also influence plant growth by its effect on activity of beneficial microorganisms. Bacteria that decompose soil organic matter are hindered in strong acid soils. This prevents organic matter from breaking down, resulting in an accumulation of organic matter and the tie up of nutrients, particularly nitrogen, that are held in the organic matter.
Changes in Soil pH
Soils tend to become acidic as a result of: (1) rainwater leaching away basic ions (calcium, magnesium, potassium and sodium); (2) carbon dioxide from decomposing organic matter and root respiration dissolving in soil water to form a weak organic acid; (3) formation of strong organic and inorganic acids, such as nitric and sulfuric acid, from decaying organic matter and oxidation of ammonium and sulfur fertilizers. Strongly acid soils are usually the result of the action of these strong organic and inorganic acids.
Lime is usually added to acid soils to increase soil pH. The addition of lime not only replaces hydrogen ions and raises soil pH, thereby eliminating most major problems associated with acid soils but it also provides two nutrients, calcium and magnesium to the soil. Lime also makes phosphorus that is added to the soil more available for plant growth and increases the availability of nitrogen by hastening the decomposition of organic matter. Liming materials are relatively inexpensive, comparatively mild to handle and leave no objectionable residues in the soil.
Some common liming materials are: (1) Calcic limestone which is ground limestone; (2) Dolomitic limestone from ground limestone high in magnesium; and (3) Miscellaneous sources such as wood ashes. The amount of lime to apply to correct a soil acidity problem is affected by a number of factors, including soil pH, texture (amount of sand, silt and clay), structure, and amount of organic matter. In addition to soil variables the crops or plants to be grown influence the amount of lime needed.
In Summary
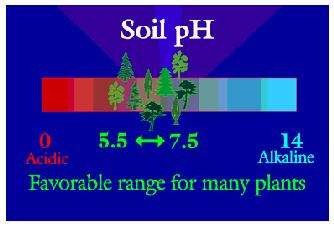 Plants are somewhat adaptable to various ranges of pH.
Plants are somewhat adaptable to various ranges of pH.
In some cases, where changing the pH would be unfeasible, it is better to find plants adapted to that pH. Your text gives a chart showing the pH tolerance range of a wide variety of plants. Broad leaf evergreens are noted for liking acid conditions. On the other end of the scale, alfalfa is one plant that likes a higher pH range.

Introduction to Acid Rain
Acid rain is a widespread term used to describe all forms of acid precipitation (rain, snow, hail, fog, etc.). Atmospheric pollutants, particularly oxides of sulphur and nitrogen, can cause precipitation to become more acidic when converted to sulphuric and nitric acids, hence the term acid rain. Acid deposition, acid rain and acid precipitation all relate to the chemistry of air pollution and moisture in the atmosphere. Scientists generally use the term acid deposition but all three terms relate to the same issue.
Precipitation is naturally acidic because of carbon dioxide in the atmosphere. The burning of fossil fuels (coal, oil and gas) produces sulphur dioxide and nitrogen oxides which can increase the acidity of rain or other precipitation. Sources of sulphur dioxide and oxides of nitrogen may be natural such as volcanoes, oceans, biological decay and forest fires, or may arise from combustion sources. The increasing demand for electricity and the rise in the number of motor vehicles in recent decades has meant that emissions of acidifying pollutants have increased dramatically from human sources, particularly since the 1950s. Emissions of such pollutants are heavily concentrated in the northern hemisphere, especially in Europe and North America. As a result, precipitation is generally acidic in these countries.
Rainfall Acidity
Rainfall is naturally acidic due to the presence of carbon dioxide in the atmosphere which combines with rainwater to form weak carbonic acid. However, the combustion of fossil fuels produces waste gases such as sulphur dioxide, oxides of nitrogen and to a lesser extent, chloride. These pollutants can be converted, through a series of complex chemical reactions, into sulphuric acid, nitric acid or hydrochloric acid, increasing the acidity of the rain or other type of precipitation, such as snow and hail.
Carbon dioxide + Water ----> Carbonic acid (weak)
Sulphur dioxide + Water -----> Sulphuric acid
Nitrogen oxides + Water ------> Nitric acid

 Plants are somewhat adaptable to various ranges of pH.
Plants are somewhat adaptable to various ranges of pH.