 Units One & Two
Units One & Two
Outline By Day
Unit I
 First Day: Thursday Feb 19 ===> a review of grade 11
Topics covered are:
First Day: Thursday Feb 19 ===> a review of grade 11
Topics covered are:
- Nomenclature; binary and polyatomic Quick list of polyatomic ions or formulae that you will encounter
- acetic acid and the acetate ion CH3COOH and CH3COO-1
- hydroxide OH-1
- cyanide CN-1
- ammonium ion NH4
- chlorate, sulphate, nitrate, phosphate and carbonate
- Rutherford Bohr theory, electromagnetic spectrum, Shells K, L, M, N, .... and the filling of these shells to elements just beyond 22.
Continuation of review, under these headings:
- Chemical/physical changes & reactions, types & balancing equations.
- Gas Laws & PV = nRT (just a quick mention, not really needed.)
- The MOLE and stiochiometric calculations; these are the ratio calculations.
- Solutions and Molarity (M) moles/litre
- A quick mention of the Thompson, Rutherford and Bohr models of the atom
- Introduction to spectroscopy and how light is produced. Photon emmission. Gas discharge tubes were viewed; emphasis on hydrogen. This is the one you must know.
 Day Two: Monday Feb 23
Day Two: Monday Feb 23
- Give out books, I hope.
- an explination of the production of the lines observed, not specific.
- Sommerfeld's mathematical improvements (Idea of using an ellipse instead of a circular orbit to describe the "motion" of an electron around the nucleus) , which leads into .....
de Brolie's wave model.
- Quantum numbers: n, l, mb, ms and diagrams with appropriate m values.
- A look at S and the three p orbitals pz, px, & py
Pictures drawn on the board, make sure you can do the same
- The concept of orbital degeneracy and a simplified look at the Zeeman effect; using a magnetic field to split orbital degeneracy
- Selection rule for quantum numbers: n>l>=|ml|
Energy level diagrams. == Electron configuration. === Zeeman effect (copied note).
 Day Three: Thursday Feb 26
Day Three: Thursday Feb 26
- Small test on what has been covered so far. Make sure you show up and are prepared to write a solid test!
- Books arrive
- The d and f orbitals
- Energy level diagram
- Making connections to the periodic table (worksheet)
- Orbital degeneracy, Hund's Rule, Aufbau principle, Pauli Exclusion principle, Heinsburger Uncertainty Principle
Homework worksheet.
- Electron configurations & Energy level diagrams; make sure you know the difference
Work on assigned text book questions. Introduction to some energy equations ( E = hf).
- Stability of half and full orbitals, electron configuration exceptions.
- Video entitled Electron Shell Structure
- Test next day
- Small assignment on paramagnetism, diamagnetism and ferromagnetism ; due next Monday March 2
 Day Four: Monday March 2
Day Four: Monday March 2
- Ionic bonding, electronegativity, ionization energy, polar bond, polar molecules, Lewis dot diagrams, covalent bonding ==> shapes of molecules
VSEPR theory and predicting molecular shapes.
 Day Five Thursday March 5
Day Five Thursday March 5
Test on Bonding
Shapes of molecules VSEPR theory
 Day Six: Monday March 9
Day Six: Monday March 9
Start of chapter four: Molecular aggragates
- In this chapter we are concerned with NOT what holds atoms together forming molecules, but what forces hold these molecules together with other molecules. If molecules did not stick together all materials would be gaseous. Hence, forces exist that hold molecules togetheforming liquids and very strong, solids. These different forces will be investigated.
- Molecular Architecture & Bonding
Definitions, Topic Overview and Central Concepts.
Forces holding atoms/molecules together with each other. Key word is INTERMOLECULAR
Bonding Forces: Strong and Weak
Strong => Ionic, Covalent, & Metallic
Weak => Hydrogen bonding, Dipole-dipole, & London or Induced dipole
Molecular forces
Molecular Aggragates
Metallic, Ionic, Covalent, Network aggragates. Network lattice structures in two and three dimensions.
 Day Seven: Thursday March 12
Day Seven: Thursday March 12
The usual Thursday test,
- Valence bond theory -- hybrid orbitals
atomic orbitals rearange and fuse together to form bonding or molecular orbitals
- VSEPR theory shapes of molecules, bond angles, names, etc
- Intermolecular forces: Strong (Ionic, covalent, & metallic) Weak (Van der Waals 3 types)
- Four types of substances (Ionic, molecular, network & metallic)
Start of Organic Chemistry
March Break is this week 16 to 20
 Day Eight: Monday March 23
Day Eight: Monday March 23
Unit Two
Organic:
Start of organic; hybridization of the carbon atom single double and triple bond formation. Use of models
The alkane family of homologous compounds
Check out the organic sites; Five of them
Start out with basic nomenclature, Alkanes, Alkenes Alkynes IUPAC naming system.
Only alkane where done today. Naming straight and cyclic alkanes. naming of substituent groups and how to number a straight chain alkane.
Video was shown, emphasis on hybridization of carbon atom
 Day Nine: Thursday March 26
Day Nine: Thursday March 26
Test on second half of Unit One; Lewis diagrams, VSEPR and Molecular aggragates.
Sigma, pi bonds with sp3, sp2, sp, hybridization. Bond rotations resulting in isomers.
Cis, trans isomers, naming chloro-alkanes, alkenes, alkynes
Preparation and reactions of the above listed series.
Make sure you know the families or classes of compounds and the functional groups.
Functional groups and how to name these compounds.
 Day Ten: Monday March 30
Day Ten: Monday March 30
Test back from Unit I; make sure you correct errors, will be taken up next day.
Simple reactions (Chapter Six), overhead note copied: tests for unsaturate bonds, formation of esters,
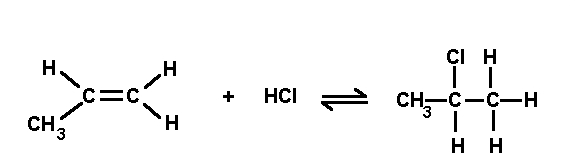
What type of reaction is this?
Use of oxidizing reagents such as KMNO4 and K2Cr2O7.
Reactions of primary, secondary and tertiary alcohols and products produced.
Experiments that should be done: an outline and results.
More information on chemical reactions; notes from overheads make sure you carefully go thru chapter 6
Polymers: Condensation & Addition , names of the six or seven most common, uses and monomers that produce them. Making Nylon 6, 10. The above listed Polymer sites looks pretty complete; check it out
Work sheet on nomenclature and reactions.
Test on organic is ????
A couple of Organic sites. Unit II stuff.
 Organic Chemistry at Portland State, Professor Wamser
Organic Chemistry at Portland State, Professor Wamser
 Day Eleven: Thursday April 2
Day Eleven: Thursday April 2
Wind-up of organic; reaction worksheet to be completed and handed in next time you come to class
Introduction to Thermodynamics, what is thermal energy, heat, temperature and calorimetry.
Heat capacity & specidic heat capacity -- what's the difference?
Heat questions using Q = mΔtc with molar heat capacity. A hand out work sheet to be taught.
Book assigned questions
Continue on to page two Page Two Units 3, 4, 5, 6
 Day Two: Monday Feb 23
Day Two: Monday Feb 23 First Day: Thursday Feb 19 ===> a review of grade 11
Topics covered are:
First Day: Thursday Feb 19 ===> a review of grade 11
Topics covered are:
 Day Two: Monday Feb 23
Day Two: Monday Feb 23 Day Three: Thursday Feb 26
Day Three: Thursday Feb 26 Day Four: Monday March 2
Day Four: Monday March 2 Day Five Thursday March 5
Day Five Thursday March 5 Day Six: Monday March 9
Day Six: Monday March 9 Day Seven: Thursday March 12
Day Seven: Thursday March 12 Day Eight: Monday March 23
Day Eight: Monday March 23  Day Nine: Thursday March 26
Day Nine: Thursday March 26  Day Ten: Monday March 30
Day Ten: Monday March 30 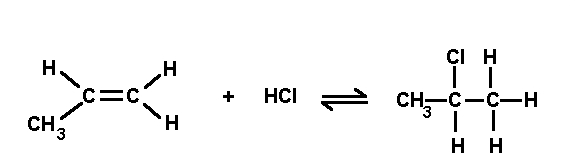
 Day Eleven: Thursday April 2
Day Eleven: Thursday April 2