 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
|
Chemistry Behind Aspirin |
|
|
|
Bonding of Compounds |
|
|
|
 |
|
|
|
|
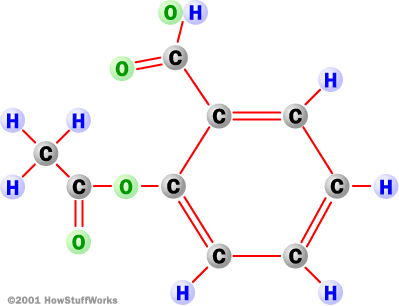
The Salysilic group, synthesized from the willow bark is composed of a Benzene ring (a hexagon made up of carbon) with Carbons doubly bonded to another Carbon and singly bonded to one other Carbon, with a total of six Carbons in the ring. Attached to four of the Carbons are singly bonded Hydrogens. On the fifth Carbon is a single bonded Carbon, who, attached to it has a double bonded Oxygen and a single bonded OH.
The last Carbon in the ring is missing 2 electrons, in order to achive a full outer shell it bonds to the Acetyl group forming a bond between the two compounds. |
|
|
|
|
|
|
Bibliography #3 |
|
|
|
But why would they bond?... |
|
|
The Salycilic Acid compound satisfies almost all of the carbons' (and other atoms) needs for an octet, however one carbon is missing an electron, so when the acetyl group comes, with the oxegyn requiring one electron, the two compounds form a bond via the sharing of two electrons. One from Oxegyn and one from the Carbon.
|
|
|
|
Bond Types |
|
|
|
Between two Carbons the bonds are covalent, non-polar. There is equal sharing of the electrons.
When the Carbons bond with Hydrogens the bond type is covalent polar, with an unequal sharing of the electrons.
The bond connecting the two compounds, the O-C, is a covalent polar bond. Due to Oxegyns higher electronegativity, it wants the electron more, this greater pull on the electron leads to is unequal sharing. |
|
|
Then the Naming .... |
|
|
|
|
|
|
When the two compounds, the Salysilic Acid and the Acetyl group combine to form one molecule, the two names also combined. The molecule is now called Acetylsalysitic Acid, or ASA. |
|
|
|
Formula...What does it mean?... |
|
|
|
The formula for ASA is C9H8O4. This formula represents that there are nine Carbons, 8 Hydrogen and 4 Oxygens in ASA.
Using this formula, we can relate back to chemistry class and find the formula mass. It happens to be 180g.
From the formula we also find that 60% of ASA is composed of Carbon, 4.48% is composed of Hydrogen and 35.5% is composed of Oxygen.
|
|
|
|
How is it Made? |
|
|
|
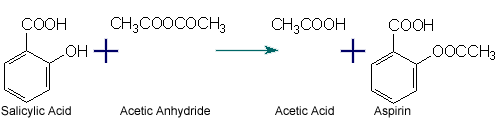
Aspirin is made by a synthesis reaction, were Salicylic Aspirin is combined with Acetic Anhydride. The products are Aspirin and Acetic Acid as a waste product. |
|
|
|
 |
|
|
|
Bibliography #6 |
|
|
|
Continue |
|

