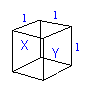
We consider a single particle in a cube, travleing in a straight line between two faces (X and Y), each time it hits either face, from the assumptions, it undergoes a perfectly elastic collision, and hence its change of momentum is m c to -m c, a total change of 2 m c. Force is equal to rate of change of momentum, so we now need to find how frequently it hits the side. It is traveling a velocity u and the cube is l accross, so there it travels 2 l before hitting the face again, this means it takes 2l / u. This makes the force on one side equal to m c2 / L, and the force per unit area (pressure) equal to m c2 / L3. Extending this for N molecules p = N m c2' / L3, where c2' is the average of the sqaure of velocity. But N m / L3 = ρ (density). Assuming that when the velocities of the molecules are divided into their perpendicular components the oscillations are evenly distributed amoung the 3 planes the pressure of a gas will be equal to a third of the density multiplied by the mean of the square of the velocity. i.e.
 IDEAL GASES The kinetic theory of pressure of a perfect gas.
IDEAL GASES The kinetic theory of pressure of a perfect gas.