Ozone is a powerful disinfecting and oxidising
agent,
successfully used in thousands of water treatment
applications.
STEVEN CARRUTHERS reports on its use in hydroponics.
What is Ozone? Ozone is a natural component of the earth's upper atmosphere, where it is primarily formed photochemically. It can be recognised by the clean, fresh odour of air after an electrical thunderstorm, which is the result of low levels of ozone gas produced by lightning.
The odour of ozone was first reported by Van Mauren in 1785, in the vicinity of an electrical discharge. In 1840, Christian Schonbein identified this charateristic odour as a previously undetermined compound. He named it ozone after the Greek word ozein, meaning to smell. Over succeeding decades several researchers experimented with the production and reactions of ozone, however the identity of the structure of the compound was confirmed in 1867 as triatomic oxygen. This simply means the ozone molecule contains three oxygen atoms, having the chemical symbol O3. Ozone is nothing more than another molecular form of oxygen, the chemical symbol for which is O2.
Ozone has been commercially used since 1893 when the first full-scale drinking water treatment application was implemented. Today, it is used in thousands of water treatment applications including the treatment of municipal water and wastewater, cooling towers, ultra pure water, marine aquaria, beverage industries, industrial process water, swimming pools, bottled water plants, aquaculture, food processing, and effluent treatment.
For hydroponic applications, ozone treatment has sometimes proved an expensive and imperfect science. However, according to Brisbane-based ozone specialist, Watertec Engineering Pty Ltd, recent work has demonstrated that ozone treatment is an effective water treatment if used correctly.
According to Technical Director Philip Barlow, ozone is the strongest commercially available oxidising agent we can produce. However, the molecule is unstable and cannot be stored for future use, as can gaseous chlorine.
"For this reason, it must be generated close to the point of application and then used immediately," he explains.
Recent field work on the Fancyleaf hydroponic farm (see article this issue) has shown that the success of ozone treatment is directly related to the volume of water in a recirculating system, the flow rate, and the number of plants the system supports. Using the Redox Potential (ORP) of the nutrient solution as an indicator, it was found that the appropriate ozone dose for the Fancyleaf operation was between 0.1 and 0.2 mg/L, requiring an ozone generator capable of delivering 5-8 grams of ozone per hour.
"If you overdose with ozone you risk damaging the nutrient solution. Also, if you don't dose sufficient ozone, the desired treatment results will not be achieved," Philip, a chemical engineer, warns.
Excessive levels of ozone will result in the loss of some micro elements in the nutrient solution, including manganese and iron.
"We're not generating enough ozone to kill all organisms; rather we're
controlling the organisms that are causing disease problems, by keeping
their numbers down," he added.
"Ozone is relatively stable in air, with a half-life of several hours at low concentrations, but this half-life changes significantly once ozone is introduced into water, where many chemical reactions can occur," Philip explains.
"Because ozone is very reactive in an aqueous environment, it can oxidise material between 10 and 1000 times faster than most oxidants used in water treatment. In some instances of organic oxidation, the material can be completely oxidised to carbon dioxide and water," Philip added.
On a comparison of oxidation strengths of standard oxidants, ozone is 2.07 volts as compared with chlorine at 1.36 volts and chlorine dioxide at 1.50 volts, versus hydrogen, respectively.
"When ozone is dissolved in water, it can react with contaminants by either direct reaction as the O3 molecule, or by indirect reaction with hydroxyl free radicals. In strong acidic solutions, the direct reaction predominates, but above pH7 the latter reaction predominates."
As can be seen from Table 1, hydroxyl free radicals have an oxidation
potential considerably above that of the ozone molecule itself. The formation
of these compounds assists with chemical oxidation, but with a very short
half life (microseconds) they do not play a major part in achieving disinfestation.
"Electrons are accelerated across an air gap, so as to give sufficient energy to split the oxygen-oxygen double bond, producing atomic oxygen," Philip explains.
The oxygen atoms which are produced by the collision react with other diatomic oxygen molecules, to form ozone according to the following equation:
3O2 + Energy = 2O3
The quantity of ozone produced is dependent on several factors, such
as the voltage and frequency of the alternating current applied to the
CD cells. When enough high energy electrons bombard gas molecules so that
they are ionised, a light emitting gaseous plasma is formed, which is commonly
referred to as a corona.
Table 1. Oxidising Potential of Various Reagents
Oxidising Reagent Oxidising Potential (V)
Fluorine
3.06
Hydroxyl free radicals
2.08
Atomic Oxygen
2.42
Ozone
2.07
Permanganate
1.67
Hypobromous acid
1.59
Chlorine dioxide
1.50
Hypochlorus acid
1.49
Chlorine
1.36
Oxygen
1.23
Bromine
1.09
Hypochlorite
0.94
"Ozone generation by corona discharge is an exothermic physio-chemical
reaction, where much of the energy used for ozone generation is lost in
heat, therefore cooling efficiency is an important factor in generator
design," Philip explains.
"Ozone destruction by decomposition increases as the gas temperature
and ozone concentration increase. For this reason, most ozone generator
designs are de-rated as the cooling water supply temperature increases.
Generator designs in which the cooling water is in direct contact with
the dielectric tubes, can tolerate higher cooling water temperatures",
he added.
Typical voltages used in CD ozonators vary between 7 and 20 kV. The
voltage required for efficient ozone generation is dependent on the generation
cell supply frequency and gap between the active electrode and dielectric
insulator.
Until recent advances with power electronics, most ozonators operated
on mains, or low frequency, being 50 or 60 Hz. For these ozonators, voltages
of between 12 and 20 kV are typical. As the voltages increase, stress on
the dielectric material also increases, which has a direct effect on the
service life of this component.
"Low frequency ozonators are still quite common and viable, however
more modern generator designs, using medium frequency (up to 1000 Hz),
offer many technical and operational advantages," says Philip.
Properly designed medium frequency ozonators are now favoured as they provide many benefits over the older low frequency technology. Some of the benefits of medium frequency ozonators include:
•Greater ozone production can be achieved with less electrode surface area. Therefore, for a given ozone output, the equipment is significantly smaller.
•When designed correctly, the power consumption per kg of ozone production is less.
•Using modern power electronics, the generator efficiency may be manipulated by varying frequency, wave form, voltage, etc.
•A greater ozonator turndown is possible and the output is very linear to frequency variations. Using both frequency and voltage control, an ozonator output of between 0-100% may be provided.
•A typical corona discharge ozone system consists of four fundamental components:
•An air preparation or oxygen production unit
•A corona discharge generator
•An ozone diffuser/contactor
•An ozone off-gas destruction system
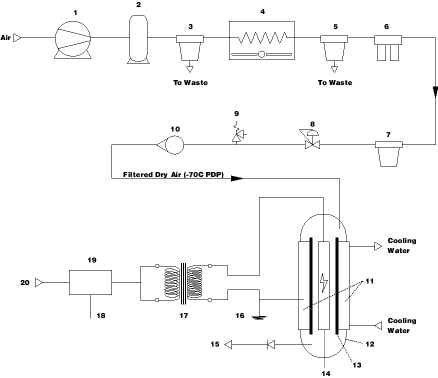
Fig 1. Typical Vertical Tube Ozonator Process Flow Schematic

1. Air Compressor 7. Dust Filter 13. Dielectric Tube 18. 4-20mA Control signal 2. Air Receiver 8. Flow Control Valve 14. High Voltage Electrode 19. Volatage/Frequency Regulator 3. Pre-Filter 9. Pressure Relief Valve 15. To Ozone Diffuser 20. 415V 50Hz Supply 4. Refrigerated Cooler 10. Air Flow Rotameter 16. Cooling Water used as 5. 0.01u Coalescing Filter 11. Cooling Water Jacket a Grounding Electrode 6. -70 C Desiccant Air Dryer 12. Ozone Generator Cell 17. H.V Transmitter
"Moist air in the ozone generator will cause nitric acid to form, which decreases the ozone production, and corrodes the generation cell components", Philip warns.
"If wet air is present, white sparks or arcing can be seen within the generator," he added.
For efficient ozone generation, the final air supply must be filtered to remove particulate matter and any oil or hydrocarbons which may carry over from the compressed air system. Ozone generators are often installed in hot, humid plant rooms. Also, many localities experience ambient conditions with both high temperatures and humidity, placing additional requirements on the air preparation system.
"Pressure fed systems are more suitable than atmospheric pressure dryers", commented Philip, "as they are able to reliably provide the necessary air quality under all normal climatic conditions."
Oxygen may be used as the ozonator feed gas, and is generally selected on the basis of higher ozone concentrations being required. The specific advantages of using oxygen is that the generator power consumption is significantly reduced, when compared to an air fed ozonator (typically 6-7 kw/kg compared with 18-20 kw/kg).
If oxygen is used for economic reasons, oxygen not converted into ozone is normally recovered, dried then recycled back to the ozonator. Due to the cost and operating requirements of these oxygen recovery systems, most applications use air as the feed gas.
Figure 1 details the air preparation typically used for CD generators,
particularly where less than ideal ambient conditions are experienced.
Fig 2. Effect of Water Vapour on Ozone Production
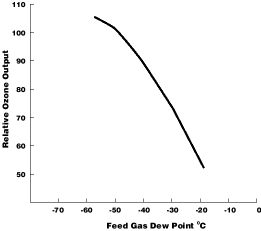
As would be expected, ozone solubility decreases with an increase in
water temperature. Also, as a consequence of Henry's Law, the solubility
of ozone increases with an increase in ozone concentration in the gas stream.
In practical applications, ozone does not have sufficient contact time
to achieve equilibrium conditions, resulting in lower solubility levels
than shown in Table 2.
Table 2. Solubility of Ozone in Water
. Ozone generated by UV
.. Ozone generated by Corona discharge
Ozone
Ozone Solubility in mg/l
conc.
%w/w
at 5°C
at 10°C at 15°C
at 20°C at 25°C
at 30°C
0.001 .
0.007
0.007
0.006 0.005
0.004 0.003
0.1 .
0.74
0.65
0.55
0.42
0.35 0.27
1
..
7.34
6.5
5.60
4.29
3.53 2.70
1.5 ..
11.09
9.75
8.40
6.43
5.29 4.04
2
..
14.79
13.00
11.19 8.57
7.05 5.39
3
..
22.18
19.50
16.79 12.86
10.58 8.09
With this system, ozone gas is normally discharged from the ozone generator at pressure of 0.7 - 1.0 Bar, which is sufficient to overcome the hydrostatic head, plus the head loss due to the gas distributor piping and diffusers. This diffusion system may also be used with ozonators operating under negative pressure, using stainless steel liquid ring gas compressors to inject the ozone, under pressure.
Some of the advantages and disadvantages of this type of system are
summarised below:

Fig 3. Typical Fine Bubble Diffusion/Contact System