Solution.
We can treat the change in diameter as a simple change in length, so we have
D = D0(1 + aDT).
The two objects must reach the same diameter:
D = D0brass(1+ abrassDT) = D0iron(1+ aironDT);
(8.753cm){1 + [19 x 10-6(C°)-1](T- 20°C)} = (8.743cm){1 + [12 x 10-6(C°)-1](T- 20°C)};
which gives
T = - 1.4 x 102C°.
Solution.
(a) If we assume argon is an ideal gas, we have
PV = nRT = (m/M)RT;
P(50.0 x 10-3m3) = [(105.0kg)(103g/kg)/(40g/mol)](8.314J/mol.K)(293K),
which gives P = 1.28 x 108Pa = 1.26 x 103atm.
(b) Because the temperature is constant, we have
P1V1 = P2V2;
(1.26 x 103atm)(50.0L) = (80.0L)P2. which gives P2 = 7.86 x 102atm.
The specific heat of mercury is 138j/kg.C°. determine the latent heat of fusion of mercury using the following calorimeter data: 1.00kg of solid Hg at its melting point of -39.0°C is placed in a 0.620-kg aluminum calorimeter with 0.400kg of water at 12.80°C; the resulting equilibrium temperature is 5.06°C.
Solution.
We find the latent heat of fusion from
heat lost = heat gained;
(mAlcAl + mwatercwater )DT = mHg(LHg + cHgDTHg )
[(0.620kg)(900J/kg.°C) + (0.400kg)(4186J/kg.°C)](12.80°C -5.06°C) =
(1.00kg){LHg + (138J/kg.°C)[5.06°C - (-39.0°C)]},
which gives LHg = 1.12 x 10J/kg.
Solution.
| (a) We can find the internal energy change Uc - Ua
from the information for the curved path a® c:
Uc - Ua = Qa®c - Wa®c = -63J - (-35J) = -28J. For the path a®b®c,, we have Uc - Ua = Qa®b®c - Wa®b®c = Qa®b®c - Wa®b; -28J = Qa®b®c - (-48J), which give Qa®b®c = -76J. (b) For the path c®d®a, work is done only during the constant pressure process, c®d, so we have Wc®d®a = Pc(Vd -Vc) = 1/2Pb(Va -Vb) = 1/2Wb®a = -1/2Wa®b = -1/2(-48J) = +24J. (c) We use the first law of thermodynamics for the path c®d®a to find Qc®d®a: Ua - Uc = -( Uc - Ua) = Qc®d®a - Wc®d®a; -(-28J) = Qc®d®a - (-24J), which gives Qc®d®a = +52J. (d) Ua - Uc = -(Uc - Ua) = -(-28J) = +28J. (e) Because there is no work done for the path d®a, we have Ua - Ud = (Ua - Uc) + (Uc - Ud) = (Ua - Uc) - (Ud - Uc) = Qd®a - Wd®a; +28J - (+5J) = Qd®a - 0, which gives Qd®a = +23J. |
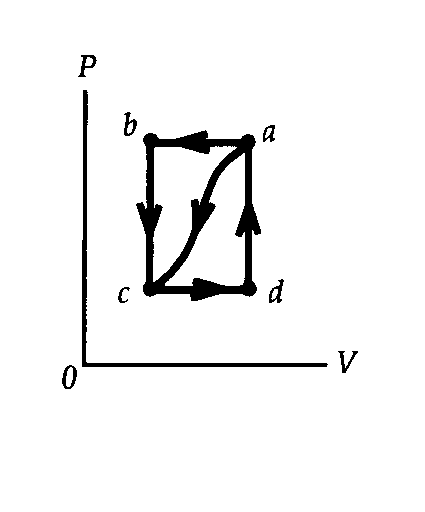 |