Microwave Chemistry
·
Home · Schedule · Tutor · Labs · Demonstrations · Chem Matters · Makeup · NotesChemMatters December 1993 Page 6
© Copyright 1993, American Chemical Society
Microwave Chemistry
by John Emsley
Microwave ovens seem to work like magic: food gets hot but the oven stays cool. It is not surprising that there are many popular myths and misconceptions about microwave cooking. One advice sheet says food should stand for a few minutes after being microwaved so the waves in the food can finish the job of heating. True of false? False! Switching off a microwave oven is like turning off a light switch — the microwaves stop instantly. But what about the following statements:
True or false?
1. Food tastes different when cooked in a microwave oven.
2. Microwaved milk is bad for babies.
3. Microwaves can turn low-grade oil into high-grade fuel.
4. Microwaves cause cancer.
5. Some people can hear microwaves.
Are they myths as well? You will find the answers on pages 8 and 9. Nearly every American home has a microwave oven. They are ideal for warming drinks, heating snacks, roasting popcorn, defrosting food, and cooking dinners. They are quick, easy to use, clean, and safe. Microwave ovens are also found in some chemistry labs, but here we need to be more careful about safety. Some simple chemicals will rocket to 1000 °C within a minute in a microwave oven. Some compounds may even explode.
What are microwaves and how do they work?
Microwaves are a type of electromagnetic radiation. Their wavelength is longer than visible light or infrared waves, and shorter than radio or television waves. They have frequencies of between 300 million and 300 billion cycles per second. (A billion cycles per second is more properly called a gigahertz, or GHz.) The frequency of all domestic microwave ovens is 2.45 GHz, chosen so the waves heat water efficiently (see side bar "Making Waves" p. 8). The power of domestic ovens is usually 700 watts.
But cooking is by no means the only practical use of microwaves! Microwaves are also used for radar control of air and sea traffic, for sending signals to satellites, and for some industrial processes. The Cha Corporation of Laramie, Wyoming, has discovered that the polluting gases from power plants can be destroyed by microwaves. Sulfur dioxide is converted to sulfur, which can be recovered for sale, and nitrogen oxides are turned into harmless nitrogen gas.
Ultraviolet light has enough energy to break molecules apart, visible light can excite their electrons, infrared waves make molecules vibrate, but microwaves can only make them spin. Even so, chemists can measure the speed at which they revolve and calculate the distances between the atoms in the molecule. Such chemists, called microwave spectroscopists, work with microwaves of higher frequencies, up to 18 GHz, than those used for domestic ovens. Microwaves can be turned to heat if they interact with a polar material and cause it to rotate. To be polar a molecule must have a positive and negative end. Water is strongly polar with the oxygen at the negative end and the two hydrogens at the positive end. For this reason water is excellent at absorbing and converting microwaves to heat, which is what happens in foods, especially vegetables, meat, and fruit that contain a lot of water. Not all water molecules can absorb microwaves. If they are held rigid, as in a block of ice, they cannot rotate and thus stay cool. You can demonstrate this by putting a cup of water and a cup of ice cubes in the microwave together for 90 seconds. The water will be almost boiling but the ice will not have melted. (If you want to try this experiment, then take the ice cubes straight from the deep freeze so they do not have a film of liquid water on the surface.)
Microwave ovens at home and in the lab
Power for microwave ovens is supplied by a magnetron, a device that generates microwaves at 2.45 GHz. These have a wavelength of 12.24-cm (4.82 inches), which means that within the cavity of the oven the waves will have regions of high energy and low energy (nodes). If we put our food at a low-energy node some of it will be poorly heated. Forth is reason some microwave ovens have a turntable so that all the food will pass through regions of maximum wave intensity. Microwave cooking does not brown food, but a browning and frying
effect can be achieved by cooking food inside packaging with "heat susceptors," which are metallized films laminated onto paper. In this way popcorn, pizzas, and meat can be made crispy on the outside. In popcorn bags the metal heat susceptor can get as hot as 250 °C (482 °F) and this explains why the bag has burn marks. (Always stay near the oven when microwaving popcorn because heat build-up can sometimes set the bag on fire.) Early microwave ovens allowed microwaves to "leak" out. In a case in which a microwave oven was being used in an analytical lab to heat samples in nitric acid, the acid fumes corroded the automatic cutoff switch. When the oven door was opened, the magnetron kept working and the technician using the oven was exposed to the waves. One day
when he stood near the oven too long the waves overheated his body and he fainted from heat stroke. Microwaves cannot escape from domestic microwave ovens, which have built-in safety devices. They cannot pass through the metal walls of the oven or the glass oven door, which has a metal mesh with holes
that are too small for the waves to get through. Even if microwaves leak out because the door seal is worn, the risks are tiny, and the heat is only a thousandth of a watt. Even if you were standing right next to such an oven you would not feel this tiny amount of heat.
Chemists began to take an interest in microwave heating after a group at Laurentian University, Ontario, Canada, showed what they could do, in 1988. Richard Gedye, Frank Smith, and Kenneth Westaway reported that reactions went faster and gave more product when they were carried out in microwave ovens. One reaction to make a compound called cyanophenyl benzyl ether took only 35 seconds in a microwave oven! Normally it would take 12 hours to complete. There were problems, however, and some of their chemical experiments exploded. Only professional chemists should use microwave ovens in the lab. Experiments must be done in Teflon bottles, and the choice of solvent is crucial. The more polar a solvent, the quicker its temperature shoots up. Nonpolar solvents such as hexane don’t even get warm; highly polar solvents such as water and methanol boil very quickly. In analytical laboratories microwave heating is routinely used for dissolving materials such as oils, coals, and biological samples by heating them in sulfuric and nitric acids, which break them down so they can be analyzed for the trace metals they contain. Chemists in these labs have commercial microwave ovens that are programmable, have built-in safety features, and cost about $10,000. These were developed by Howard Kingston of the National Institute of Standards and Technology, Washington, DC. Metals should never be put into microwave ovens because they will generate an electric current and cause sparks. Graphite, which also conducts electricity, will glow white hot in a microwave oven. Methane gas will spin off its hydrogen atoms, leaving bare carbons, and these can be condensed onto surfaces to form diamond films. Perhaps the most unexpected effect of microwaves is seen when heating simple metal oxides such as those of copper and zinc. The temperature of a five-gram sample can jump ten degrees a second so that within a minute these oxides have melted. Superconducting mixed metal oxides, such as yttrium barium copper oxide, can be made within minutes in a microwave instead of the usual method of heating for more than 24 hours. (See "Superconductivity," Chem Matters, October 1987.)
Microwave myths—true or false
From its earliest days microwave cooking led to popular myths. How did you answer the questions on page 6?
1. Food tastes different when cooked in a microwave oven: true
Takayuki Shibamoto and Helen Yeo of the University of California, Davis, researched the volatile molecules that cooking produces, which give cooked food its attractive flavor. They found that conventional cooking produces more of the desirable flavors such as thiazole, furan, and pyrazine whereas microwave cooking produced less desirable flavors such as oxazole, thiophene, and pyrrole. They blame shorter cooking times and lower temperatures for these chemical differences, but say that adding salt before microwaving food can enhance the production of the more desirable flavors. However, salted food may not cook as well because hydrated salt ions act as polar molecules and absorb the microwaves at the surface of the food. The result is that the interior of the food remains cool. Varoujan Yaylayan of McGill University in Montreal has developed a solution of amino acids and sugar with which foods such as chicken can be coated before being microwaved, to produce a freshly roasted flavor. 2. Microwaved milk is bad for babies: false
It is true that microwaving milk converts some amino acids such as L-proline into D-proline, which babies cannot digest. However, research by Leonard Petrucelli and George H. Fisher of the Department of Chemistry at Barry University, Miami, Florida, found that heating cow’s milk on an ordinary hot plate caused exactly the same chemical changes.
3. Microwaves can turn low-grade oil into high-grade fuel: true
Exxon Research and Engineering of New Jersey has patented a process that will turn uneconomic shale oil and tar sand, of which there are vast deposits in the world, into useful hydrocarbons and ethylene gas by inserting wires and bombarding it with microwaves. The microwaves cause the wires to discharge electricity, which converts the crude oil or tar to high-grade fuels.
4. Microwaves cause cancer: false
Quite the reverse: microwaves can kill cancer. Hyperthermia treatment uses microwaves to heat cancer cells within the body, and because such cells do not have a network of blood vessels they cannot cool themselves and they die. Cancers in the breast, neck, and head can be treated this way. A few years ago there was a scare that microwaves might cause cancer, when study on laboratory rats exposed to low-energy microwaves for long periods of their lives showed the development of slightly more chromosome damage. This work was published in 1984. However, other scientists who looked at the data were not convinced and further research failed to support the findings. A few years ago it was discovered that certain plastics, such as cling film and containers, released molecules that were absorbed by the food, especially by fatty foods. Some of the released molecules were suspected of causing cancer. Since then manufacturers have reformulated plastics such as PVC to prevent this form happening, but in any case the formulations used to make plastics flexible, colorful, and fire-resistant have been tested and only those that are perfectly safe are now used. (See "Risky Business" on page 10 of this issue.)
5. Some people can hear microwaves: true
Low-energy microwaves can generate sound waves inside the human skull; some people experience this as a clicking noise. It is not dangerous and is not produced by the higher energy microwaves that are used for cooking.
SIDE BARS
Making waves
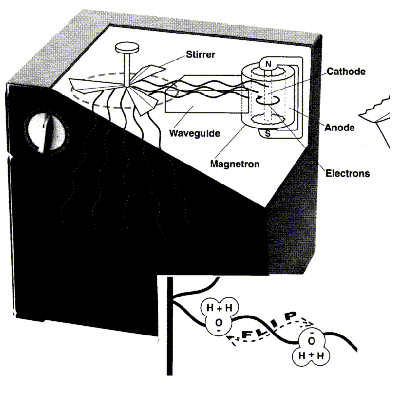
Microwaves are generated in a device called a magnetron, a World War II invention that was the heart of antiaircraft radar. The magnetron is a hollow cylinder with irregular walls, a rod-like cathode in the center, and a strong magnet positioned with N and S poles at opposite ends of the cylinder. An electric current flows from the cathode (which is electrically heated to help free electrons), across the air space, to the cylinder wall that serves as the anode. As electrons begin this passage, the magnetic field forces them to move in circles around the cathode. The circular acceleration of the charged electrons creates
electromagnetic waves. The magnetron in ovens is designed to produce waves that vibrate 2.45 billion times a second, that is, at a frequency of 2.45-gigahertz (GHz). Called microwaves because of their relatively short length (12 cm), the waves flow through a pipe-like guide to the stirrer, which looks like a fan but acts to reflect the microwaves in many directions. The microwaves bounce off the metal walls of the oven and strike the food dish from many angles. The waves pass through dishes made of glass or plastic with no effect, but strongly affect mobile, charged particles (such as dissolved ions or polar molecules). As each wave crest passes a polar molecule, the molecule is forced to turn and align with the wave. A moment later, as the trough of the wave passes, the molecule is turned in the opposite direction. Water is the most common polar molecule in food and the microwaves vibrate at 2.45 GHz because this is close to the optimum rate for making H 2 O oscillate
The friction of the back-and-forth oscillation heats the water and the surrounding food. Food is generally not heated above 100 °C in a microwave oven because, as water boils away, it takes heat with it. Popcorn, however, must be heated in oil above 200 °C, though oil is not heated by microwaves as effectively as water. The food technologists who developed microwave popcorn solved this problem by adding a piece of metal foil (or metal-coated plastic film) to the paper bag. Microwaves are reflected by large metal surfaces but may be absorbed by small metal objects. The microwaves induce electric currents to flow back and forth through the metal, which quickly heats the foil far above 200 °C.
Popcorn kernels contain starch, protein, and water sealed within a tight hull. Surrounded by approximately 260 °C oil, the kernel heats up quickly and the water and steam within rises far above the usual boiling point of water because the sealed hull keeps the contents under pressure. The pressurized steam transforms the starch grains into hot, gelatinized globules. When the hull finally ruptures—at 175 °C and a pressure of 9 atmospheres—the expanding steam inflates the starch into the fluffy white foam that we love to eat with butter and salt. (See "Popcorn," Chem Matters, October 1984.)
From secret weapon to magic oven
Microwave cooking was discovered in the fight against the Nazis in World War II. At the start of the war in 1939 the Royal Air Force used microwaves to track Nazi warplanes attacking England. They called their secret rays radar. The electronic tubes they used to produce the rays were manufactured secretly by the American company Raytheon at Waltham, Massachusetts. There the radar testers and operators discovered that cups of coffee that had gone cold could be warmed by placing them near the electronic tubes—and so microwave cooking was born. The first microwave ovens were made by Raytheon in 1946 and cost about $1500 (more than $20,000 at today’s prices!). They were expensive because they were large and needed a water supply to cool the electronic tubes.
A better way of generating microwaves was discovered by an ex-engineer of Raytheon, John Gunnarson, in the 1950s. This was the klystron valve, but he never lived to see its use in modern microwave ovens. The real breakthrough in a heap ovens was made by Keishi Ogura of NJR of Japan. He worked on the magnetron and came up with a version that was cheap, reliable, did not need to be cooled, and had a long life. By 1980, oven magnetrons were being made by Toshiba for only $7, and the price of domestic ovens today has fallen to less than
$200.
BIOGRAPHY
John Emsley is the Science Writer in Residence in the Department of Chemistry, Imperial College, London.
REFERENCES
Emsley, J. "The chemist’s quick cookbook"; New Scientist 1988, 120, (1638), 56. "Ordinary kitchen microwave can speed organic reactions"; The Science Teacher 1992, 59 (4), 14.
Pagnota, M.; Nolan, A.; Kim, L. "A simple modification of a domestic microwave oven for improved temperature control"; Journal of Chemical Education 1992, 69 (7), 599.
Roman, M. "The little waves that could"; Discover 1989, 10 (11), 54.
![]()