|
[HOME] |
THOMAS A. POWERS
RESEARCH PAPERS
[RESUME] [RESEARCH PAPERS] [PATENTS]
I) Oxygen-17 NMR Spectroscopy of Sulfones
|
Oxygen-17 nuclear magnetic resonance spectroscopy of organosulfur compounds. Part III. Oxygen-17 NMR lanthanide-induced shifts (LIS) of diastereotopic oxygen atoms in trans-2-[alkyl(aryl)sulfonyl]cyclohexanols
By Thomas A. Powers and
Slayton A. Evans, Jr.
Abstract: The 17O
NMR diastereotopicity or chemical shift
|
|
|
Oxygen-17 nuclear magnetic resonance spectroscopy of organosulfur compounds. 2. 17O NMR lanthanide-induced shifts (LIS) of diastereotopic sulfonyl oxygens in substituted six-membered-ring sulfones
By T.A. Powers; Slayton A.
Evans, Jr.; K. Pandiarajan; and J.C.N. Benny Abstract: The 17O NMR shifts of diastereotopic sulfonyl oxygens within a series of conformationally homogeneous six-membered-ring organosulfur compdounds have been determined. Their lanthanide-induced shifts, resulting from competitive complexation with the europium metal ion [i.e., (Eu(FOD)3], provide structural insights into the relative binding potential of the attached diastereotopic sulfonyl oxygens.
|
|
|
Oxygen-17 NMR studies of heterocyclic sulfones and trans-2-(alkylsulfonyl)cyclohexanols utilizing the lanthanide shift reagent Eu(FOD)3
By Thomas A. Powers; Lee G.
Pedersen; and Slayton A. Evans, Jr. Abstract: Complexes between paramagnetic metal ions and sulfones bearing diastereotopic oxygens are examined through 17O NMR spectroscopy of heterocyclic sulfones and trans-2-(alkylsulfonyl)cyclohexanols in the presence of Eu(FOD)3, as well as through ab initio calculations on Lithium complexes of 3,4-epoxythiolane 1,1-dioxide.
|
|
|
Lanthanide induced oxygen-17 NMR shifts of diastereotopic oxygen atoms in 1-thiadecalin 1,1-dioxide and related compounds
By Thomas A. Powers and
Slayton A. Evans, Jr.
Abstract: Lanthanide-induced 17O NMR shifts of diastereotopic sulfonyl oxygens in 1-thiadecalin 1,1-dioxides (shown at left; X = CH2, O, or S) provide a basis for determining equilibrium constants between diastereomeric Ln-sulfone complexes.
|
II) Organic Electrochemistry
|
Electrochemical reduction of (1-bromo-2,2-dimethylpropyl)benzene in dimethylformamide on carbon electrodes
By Albert J. Fry and Thomas A. Powers Abstract: Products from the electrochemical reduction of PhCHBrCMe3 on carbon electrodes in DMF containing lithium perchlorate depended upon the electrolysis potential. At relatively positive potentials the products are derived primarily from the coupling of two benzylic radicals, as shown in Scheme 1:
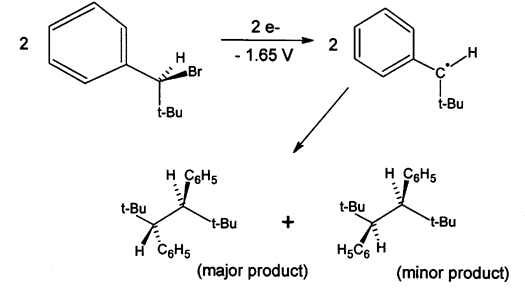
At more negative potentials the products are derived from the corresponding carbanions, as shown in Scheme 2:
Scheme 2 This establishes for the first time the mechanism of bibenzyl formation in the electrochemical reduction of benzyl bromides at nonmetallic electrodes. The meso-dL ratios of the 1,2-di-tert-butyl-1,2-diphenylethane products are dependent upon electrolysis potential; head-to-head coupling of .CH(CMe3)Ph is sterically restricted.
|
|
Electrochemical behavior of layered annulenes
By Albert J. Fry; Thomas A.
Powers; Klaus Muellen; and Wolfgang Irmen
Abstract: Reduction
potentials of [14]annulenes (I; n = 3-5; II, III) were determined by
cyclic voltammetry. The voltammograms of I (n = 3, 4) exhibit clear
evidence of electronic interaction between the 2 pi systems. ![Layered [14]Annulenes](images/compound.gif)
|
[MY TRAVELS] [MY LIFE] [MY CAREER]
[LINKS] [SITE MAP] [SIGN GUEST BOOK] [VIEW GUESTBOOK] [WHAT'S NEW]