 Back to home.
Back to home.Here I will show you in what way we can describe the behaviour of a very curious, yet very common type of matter in the universe: a plasma. If you want to know more just send me an e-mail.

We probably all heard about the existence of molecules and atoms. And when you haven't you can learn more at the other topics of my webpage. These molecules and atoms are the building blocks of matter and we can see the result of that building everywhere around us, from the human body to the Himalaya mountains. One might therefore be tempted to say that this type of matter must be the most common form of matter in the universe.
However, there is one big gap between the world of the tiniest particles and the world on the large cosmological scales where only the force of gravity seems to play an important role. Why is there nothing in between? Is it just that we are the only ones that can see it? And we are simply not operating on such a large scale. That sounds somewhat ludicrous to someone unwilling to attach such a grave importance to the human race.In fact, 90% of all matter contained in the visual universe is in a special state of matter, which we call "plasma". The word originally stems from the old Greek language, meaning "living beast". A word quite appropiate as will be understood after reading this page. Now, of course you think you are not very familiar with the concept of a plasma state of matter, but I will give some examples of plasma's that might give you an impression of what a plasma is. The real theoretical way of describing a plasma will start after that.



You probably recognize the first two pictures and some of you maybe even the last one as well. Anyway, the first is a neon lightbulb, the second a picture of lightning and the last one is a picture taken by SOHO, an ESA sattelite circling the Sun and taking various picture of the upper atmosphere of the Sun. The centre of the Sun is blocked by a black screen in the central part of the camera, since all the light coming from the direct surface of the Sun, would make the higher atmosphere of the sun become invisible. That's way on Earth we can never see the Sun like this, except when there is a solar eclipse. (By the way, just for the fun, do you realize how incredibly coincidental it is that the distance of the moon to the Earth and the Earth to the sun is so that during an eclipse the moon EXACTLY covers the sun?)
Of course the plasmas you just saw still consist of elementary particles, however, there are some very curious things happening with the particles in the plasmas. For instance, looking at all the plasmas they seem all to act like a kind of fluid or blub. That's no coincidence, a plasma indeed follows the same equations as any fluid or gas does. They also seem to be quite energetic, radiating with a lot of light. This is no coincidence either, since there are some special properties concerning the particles in the plasmas that require a large amount of energy being put in the plasmas. Finally look at the difference between the three plasmas. The lightning follow quite a herratic, unorder path towards the Earth, however, the plasma inside the lightbulb seems to be perfectly confined withing the glas casing and, for the slightly trained eye, the plasma forming the upper part of the Solar atmosphere is confined as well. But of course not by some rigid wall or anything like that. How can we understand phenomena involving plasmas? And what importance do they have for our community? I will try to answer those two questions at first in the most basic mathematical and physical picture. The exact description of the behaviour of plasmas needs a large amount of rather complex mathematics, which I might include after I have finished the most basic description.Ordinary matter can be present in different forms under different circumstances. For instance, take water, one of the most abundant and biologically important substances of all. Under atmospheric pressures (1 Bar) and roomtemperature (20 degrees C or 293 K) water is a fluid. Lowering the temperature below 0 degrees C (273 K) water will sollidate into ice. Increasing the temperature above 100 degrees C (373 K) will vaporize the water into gas.
These three forms are best known to man. It is important to realize that different substances have different temperatures (at equal pressure) where they will vaporize, melt or sollidify. For instance, the existence of high percentage alcohol is due to the fact that alcohol vaporizes at lower temperatures than water does. (about 43 degrees C) So one can seperate alcohol and water, by simply heating the mixture and catching the first damp coming from the mixture. It is more important however to realize that even though the results of measurements are different, the underlying physics is the same.
The most familiour form of matter is probably that of a solid, like wood, iron, stone etc. Solids consist of molecules or atoms, which are all inside strictly organized groups. Some come in the form of crystals, for instance kitchensalt (NaCl, or Sodiumchloride). Others form big conglomerations of small crystals like grapphite, the stuff the top of your pencil is made of. The thing the solids have in common is that their building blocks, the atoms, are unaible to move freely. The are packed in a rigid pattern and the only degree of freedom they have is to vibrate around their average position inside this pattern.
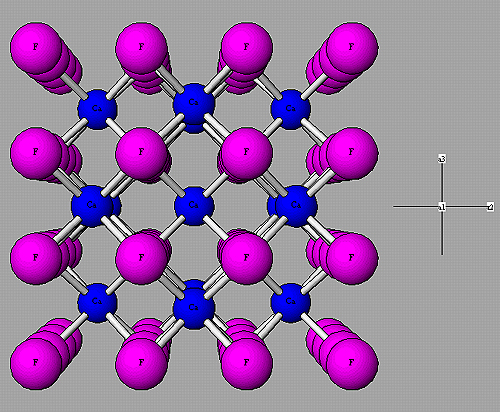
This kind of structuring in very symmetric groups is also the reason why the crystals of, for instance salt, show such regular large-scale structures. It is not very strange that so many substances seem to be in the form of a solid, at least on Earth. Physical systems tend to be in that state which has the lowest energy and confining the atoms to certain places removes their ability to move around freely and thus lowers their kinetic energy enormeously. However, this does not explain why different substances have different melting points.
That really has to do with the different atoms a substance is made of. Different atoms have different amounts of electrons orbiting the chore, which are in different electron orbits having different energies. The temperature at which a solid melts is really just a result from the different in bonding strengths of the electrons.
Now, what happens when we heat a solid slowly? The atoms will gain energy and will start to vibrate with larger and larger amplitude. At some point the vibrational energy might overcome the bonding energy and the atoms gains degrees of freedom. The total energy of the atom is now divided over more directions in which the atom can move. The velocity in one direction might therefore not increase much, but the average velocity will gain.
At some point the atoms will be all freed from their shackles of the crystal, but will still not have enough total energy to be moving completely freely. They are sort of rubbing against each other, move with relatively low velocities. This state is called the fluid state.
