

VINAY"S CHEMISTRY REVISION NOTES IGCSE 2004 CHEMISTRY
BACK TO MAIN PAGE BACK TO CHAPTER INDEX CONTACT ME: vinz@india.com
Chemistry IGCSE Chapter 3 Atoms, Elements and Compounds
The Atom
· Smallest particle that can take place in a chemical reaction
· Consists of three sub-atomic particles, electrons, protons, neutrons
Electrons
· Negatively charged, found in energy shells outside the central part of the atom
· Move at very high speeds in orbit
· Have very negligible mass
· Mass is 0.0005 of a proton
Protons
· Are positively charged and found in the nucleus of an atom
· Have a unit mass of one (ie. 1 a.m.u)
Neutrons
· Have no charge at all and are located in the nucleus
· Have a unit mass each
1 atomic mass unit (1 a.m.u) = 1.67 x 10-27 kg)



Energy Shells
· Shells increase as they become full
· The first shell only contains 2 electrons and following shells contain 8 each
Chemical Stability
· Electrons are spaced out regularly at intervals as shown below. The presence of unpaired electrons determines the chemical reactivity of the atom.
· The electrons in the outer shell of an atom are referred to as valence electrons
· If the valence electrons for the outer shell are not enough to fill the shell, then the atom is chemically reactive and unstable. (E.g. Sodium Na+)
· Atoms with fully filled outer shells such as noble gases, are chemically unreactive and stable.
Noble Gases
· Also called inert gases have fully filled outer shelled atoms (E.g. Helium, Neon, Argon, Krypton)

Electronic Configuration
· A group of numbers which show the arrangement of electrons in their various shells
· The numbers represent number of electrons in each shell starting with the innermost shell, separating each number (shell) by a comma
E.g. He: 2, Ne: 2,8, O: 2,6
Isotopes
· Atoms of the same element having different mass #s but the same atomic number (ie. Different neutrons)
· Most elements exist as a mixture of their isotopes
· Hydrogen has 3 isotopes: Protium (0n), Deuterium (1n) and Tritium (2n).
These are called H-1, H-2 and H-3
The two types of isotopes are: (i) Radioactive and (ii) Non-radioactive isotopes
· Radioactive isotopes are isotopes with an unstable nucleus (the general term for radioactive substances since they are all isotopes).
· Non-Radioactive isotopes are stable and unreactive
Medical uses:
· Isotope cobalt-60 emits gamma radiation which can be used to sterilize medical equipment
Industrial Uses:
· Uranium-238 can be used to estimate the ages of rocks
Differences between Elements, Mixtures and Compounds
· An Element is a pure and simple substance which cannot be broken down into any simpler substances other than itself by any ordinary chemical means.
· A Mixture is made up of two or more substances physically mixed together.
The components in a mixture can always be separated by physical means
When these combine chemically they lose their identities and the compound takes its own properties.
|
Mixture |
|
Compound |
|
No heat or light is given out or absorbed when mixing occurs |
|
Heat and sometimes light is usually given out or absorbed when a compound is made. |
|
The substance in a mixture can be separated by physical means |
|
the elements in a compound cannot be separated by physical means |
|
the properties of a mixture are the average of the properties of thes substances in it |
|
the properties of a compound are quite different from those of the elements in it |
|
the substances in a mixture can be presented in any proportions by mass |
|
the elements in a compound are combined together in definite proportions by mass (we have not proved this yet). |
|
Metals |
|
Non-Metals |
|
good conductors of heat and eletricity |
|
poor conductors of heat and electricity |
|
shiny |
|
dull |
|
malleable |
|
brittle |
|
strong |
|
weak |
|
react with oxygen to form basic oxides |
|
react with oxygen to form acidic oxides |
|
usually high density |
|
usually low density |
|
usually high melting points except alkali |
|
usually low melting points except carbon |
|
many react with dilute acid to produce hydrogen |
|
no reaction with dilute acid |
Alloys
|
Name of Alloy |
Composition |
Uses |
|
Brass |
Copper, Zinc |
To make musical instruments (especially wind instruments). Utensils |
|
Bronze |
Copper, Tin |
Utensils, metal, statues, decorating pieces of art works |
|
Duralumin |
Aluminium, magnesium, copper, manganese, silicon |
manufacturing parts of aircraft (e.g. the wings) |
|
Steel |
iron, carbon (and sometimes manganese) |
machine parts, cooking utensils |
Ion Formation
· Atoms that have more than ½ the max # of electrons in their outer shell (ie. 4 +) will tend to gain electrons needed to make 8 (an octet) and hence a stable atom
· If less than ½ then it will tend to lose electrons
· 17Cl has 17e (Cl: 2,8,7) and requires one electron to achieve chemical stability

· The new atom has one electron in excess, compared to protons. It will thus carry an extra charge of 1 (minus) and becomes Chloride Ion.
· Ions with negative charges are called anions
· Similarly, metals, having less than 4 valence electrons, will tend to lose electrons to become stable.
· This way they end up having an excess +ve charge.
· Positively charged ions are known as cations.
· Ions are electrically charged particles formed when atoms lose or gain one or more electrons to form a stable outer shell.
Bonding
· A chemical union developed due to attraction when atoms lose, gain or share electrons in order to acquire stable (full) outer shells.
· Atoms are held together by bonds
· Bond is formed by the use of a valence electron (ie. The electron in the outer shell of an atom.)
· Types are (i) Ionic (ii) Covalent (iii) Metallic
Ionic Bonding
· The attraction between the positive and negative ions (anions and cations) formed by loss/gain of electrons (ie. Metals and non-metals)
· This attraction results in a chemical union known as bonding
· The union involves transfer of electrons
· It is exhibited by elements far apart in the periodic table (metals and non-metals)
· In the formation of sodium chloride (Na+Cl- are the ions in the compound) the metallic element (sodium) burns in chlorine (the non-metallic element).
· Sodium atom has 1 valence electron and will tend to lose this. Each sodium atom will lose on electron to become a sodium ion. Similarly, Chlorine has 7 valence electrons and needs to gain one to become chloride ion.

· Sodium atom outer shell and Chlorine outer shell now both contain 8 electrons. However, the two atoms are now bonded and share opposite charges. They is hence and electrostatic force between them holding them together.
·
 This electrostatic force is an
ionic bond or electrovalent bond.
This electrostatic force is an
ionic bond or electrovalent bond.
· Electrovalent bonds exist between electrovalent compounds. These compounds can conduct electricity when melted or dissolved.
· Magnesium Fluoride is also an ionic compound
·
 Magnesium (2,8,2) has two electrons in its
outermost shell. It needs to lose these to gain a stable, full outer shell.
Fluorine needs to gain one electron. One magnesium atom needs two fluorine atoms
to accept two electrons. Formula is Mg2+2F- or MgF2.
Magnesium (2,8,2) has two electrons in its
outermost shell. It needs to lose these to gain a stable, full outer shell.
Fluorine needs to gain one electron. One magnesium atom needs two fluorine atoms
to accept two electrons. Formula is Mg2+2F- or MgF2.
Giant Ionic Structure
· The attraction between opposite charged ions results in a three-dimensional structure of alternate +ve and ve ions.
· This is a crystal of the ionic compound
· Since they cannot move out of their position, the ions cannot conduct electricity however, when the compound melts, the 3d structure breaks down and the ions can move towards the electrodes
· Structures of ionic solids are called giant ionic structures
Properties of Ionic Compounds
· Crystalline solid with high melting and boiling points (due to strong bonds)
· Conduct electricity in molten (fused) form or aqueous solution
· Most are soluble in water
· Non-volatile
Covalent Bonding
· The sharing of electrons pairs between atoms (non-metals) so that each atom acquires a stable outer shell. Two electrons (a pair) shared is an electron pair.
· A covalent bond is represented by each electron share
· Covalent bonds are strong and involves only the outer shell of atoms
· In most cases, it takes place when there are 4 atoms in the outer shell and so the atom can only share.
· In most cases, the energy shells overlap such that electrons from one atom can orbit the other.
Formation of H2 Gas (single bond):

Formation of Cl2 Gas (single bond): Formation of H2O:


Formation of HCl:
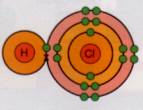
 Formation of CH4:
Formation of CH4:
Double Covalent Bonds
· When two pairs of electrons are shared
· Formation of CO2
· Two oxygen atoms required one carbon. Each oxygen atom shares 2 electrons with the carbon atom and in turn receives two electrons. Full outer shells for both elements.

Covalent Compounds/Giant Molecular Covalent
· Usually liquids or gases at room temp.
· Consist of molecules that vaporize easily volatile
· Often have distinctive smells
· Many insoluble in water bust soluble in organic solvents (ethanol, chloroform)
· Melting and boiling points are low (weak attraction between bonds)
· Do not conduct electricity because they are not ions present

Metallic Bonding
· Attraction between particles in a giant metallic lattice
· Bonding metals and metals
· Lattice consists of positive ions of the metal with valence electrons (delocalized) free to move between them.
· Heat and electricity can be conducted through the metal because of the free/delocalized electrons moving from the bonds between
· The negative electrons and positive ions cause strong electrostatic force, metals have high melting and boiling points
· Electrons try to escape, and increase positive charge of ions, which are attracted back 2 lattice.
· Layers of atoms can slide over on another, making metals malleable and ductile.
Diamond and Graphite
· Allotropy: the existence of an element in more than one form without change of state.
· Carbon exists in more than one form in the same state
· The three forms in which carbon occurs are: diamond, graphite and amorphous carbon (charcoal etc.)
· Polymorphism: the existence of substance which can crystallize in more than one form (without change of state)
· Polymorphs of carbon are diamond and graphite
Diamond
o Colourless, transparent and sparky
o Each carbon atom joined by covalent bonds to four others arranged in tetrahedral form
o Each diamond structure is therefore a giant molecule containing very strong covalent bonds
o Strength and uniformity of bonding in all directions makes diamond very hard
o No mobile electrons all 4 valence electrons used in covalent bonding (cannot conduct electricity)
o Has high melting pt. because consists of structures of carbon atoms
 Graphite
Graphite
o Macromolecular crystal of graphite consists of layers of carbon atoms (each layer made up of an extended network of carbon atoms in a hexagonal form)
o Within a layer, carbon atoms are held together by strong covalent bonds
o The layers are joined together by weak intermolecular forces.
o These forces are easily broken and the layers move relative to each other (soft + slippery nature of graphite)
o Each carbon atom bonded to only three others in layers. The 4th valence electrons from each atom are free to move from 1 hexagon to the next within a layer and thus conduct electricity
o High melting point due to giant structures of carbon atoms. (used as electrodes)

Silicon (IV) Oxide (SiO2
o
 Not a dioxide because its crystals consist
of giant molecules in three dimensions in which each silicon atom is bonded
tetrahedral to form oxygen atoms.
Not a dioxide because its crystals consist
of giant molecules in three dimensions in which each silicon atom is bonded
tetrahedral to form oxygen atoms.