Description of Clinical Trials
Treatment of Hyperopia and Astigmatism
Source Site (EDGAR):
424B1
Excerpt From
KERAVISION INC /CA/
Form: 424B1 Filing Date: 8/13/99
|
424B1
FINAL PROSPECTUS DATED 08/12/1999
26
Expand our existing product portfolio
We intend to develop additional products to treat a broad range of vision
disorders based on our Intacs technology. For example, we are currently
conducting a U.S. Phase III trial for a product to treat myopia in both higher
ranges (-3.1 to -4.5 diopters) and lower ranges (-0.75 to -1.0 diopter). In
addition, we have recently initiated a second feasibility trial outside the
United States for our initial hyperopia product based on the results we
achieved in an earlier trial. We have also successfully treated a small number
of patients with pure astigmatism and are in the early stages of development
with a product designed to treat myopia concurrent with astigmatism greater
than +1.0 diopter. We have designed these products to retain the benefits of
our Intacs technology including maintaining the integrity of the central
optical zone and the option of removability.
27
Astigmatism. KeraVision has studied a potential product that uses arc
segments to treat pure astigmatism. This device utilizes the core Intacs
technology for the treatment of myopia and the segments are manufactured from
the same material as Intacs. A similar surgical technique is used to implant
the segments in the eye. In August 1994 and March 1995, KeraVision implanted
arc segments in eight astigmatic patients. Clinical results showed that these
patients exhibited improvement in their vision. KeraVision is pursuing broader
experimentation and analysis, but has not enrolled further patients due to the
perceived smaller size of the potential market compared to the markets for
myopia and hyperopia.
-------------------------------------------------------------------
Source Site (EDGAR): 1999 10-K
Hyperopia. Treatment for correction of hyperopia consists of implanting six rectangular
segments made of PMMA placed in the periphery of the cornea. These segments steepen the center
of the cornea and flatten the periphery. Each segment is 2.0 mm long and 0.8 mm wide, with a
thickness ranging from 0.25 to 0.45 mm. Patients requiring +1.0 to +3.0 diopters of hyperopia
correction have experienced significant improvement in their vision. After 12 months following
the placement procedure, 40% of patients achieved vision of 20/20 or better, 90% saw 20/25 or
better, and 100% saw 20/40 or better. Because only a limited number of patients to date have
received the treatment for hyperopia, KeraVision will need to treat additional patients to
characterize the range and predictability of correction prior to moving to a large scale
trial. We have initiated a second feasibility trial at several additional sites in Europe.
-----------------------------------------------------------------------
Source Site (EDGAR): 8-K
TYPE: EX-99.30
SEQUENCE: 2
DESCRIPTION: NEWS RELEASE DATED 8/27/1999
Exhibit 99.30
In another clinical study for potential new Intacs products,
a fifth clinical site has been added to KeraVision's European hyperopia
feasibility trials. Josep Guell, MD, of the Institute of Ocular
Microsurgery in Barcelona, Spain, will use Intacs to treat up to 20
patients for mild and moderate farsightedness (+1.0 to +5.0 diopters of
hyperopia). Other Intacs hyperopia study sites are in Austria and
Germany. As previously announced, KeraVision plans next year to apply
for "CE" mark approval to sell Intacs for hyperopia in the European
Union countries.
_____________________________________________________________________
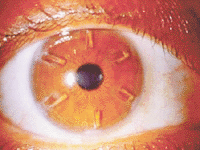 |
Intacs for Hyperopia |

