| They are made of PMMA, a material that has
been used for nearly 50 years for contact lenses and IOLs after cataract
surgery. Intacs are designed for permanent placement in the eye, but they may
be removed or exchanged if necessary.
The KeraVision Intacs are currently approved for the correction of up to
3.50 D of myopia. Ideal candidates are those with less than 3.00 D. In terms of
success, no other procedure to date has completed FDA studies with a greater
percentage of patients better than 20/20. At Hunkeler Eye Centers in Kansas
City, Mo., data from Daniel S. Durrie, MD, show that 75.6% of the patients were
20/20 or better and 53% were 20/16 or better. Intacs appeared to have the
quickest visual recovery of any procedure to date.
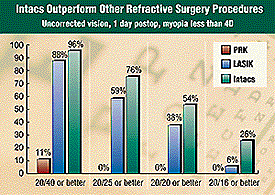 --Fast recovery: Intacs have a
faster visual recovery, with more patients achieving 20/25 and 20/20 or better
visual acuity sooner than PRK or LASIK. --Fast recovery: Intacs have a
faster visual recovery, with more patients achieving 20/25 and 20/20 or better
visual acuity sooner than PRK or LASIK.
In the accompanying bar graph, we compare patients with this same
refractive error (–1 to –4 D) for photorefractive keratectomy (PRK),
laser in situ keratomileusis (LASIK) and Intacs. We see that Intacs have a
faster visual recovery, with more patients achieving 20/25 and 20/20 or better
visual acuity sooner than PRK or LASIK.
![[bar]](osnart/gradient.gif)
Implanting Intacs
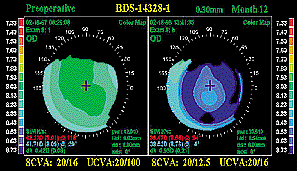 ---Reversible procedure: The topographic
maps at the top show that the surgical treatment does not reverse the natural
positive corneal asphericity. The maps on the bottom show the cornea
preoperatively and 3 months after removal, indicating that the corneal
topography returned to its original state. ---Reversible procedure: The topographic
maps at the top show that the surgical treatment does not reverse the natural
positive corneal asphericity. The maps on the bottom show the cornea
preoperatively and 3 months after removal, indicating that the corneal
topography returned to its original state.

The procedure takes about 5 to 15 minutes to perform and begins with a small
(1.8 mm) incision at the 12 o’clock position outside the central optical
zone. Like LASIK, the eye is held in place with a suction ring. A specially
designed blunt instrument is used to separate the layers of the stroma,
creating a semi-circular tunnel within the corneal stroma. First one segment is
rotated into position, then the other. Once the segments are in position, the
procedure is performed on the other eye. The Intacs correct myopia by
flattening the central corneal curvature.
Patients may describe discomfort when suction is on during the surgical
procedure just as in LASIK. Post operatively, some patients describe some
burning, foreign body sensation or even photophobia that lasts for about 2 to 4
hours. Because the Intacs are placed at two-thirds depth in the corneal stroma,
patients usually cannot feel the Intacs in the eye unless they rub the eye
vigorously. Although some patients may initially notice a foreign body
sensation after the procedure, they should be instructed that this is part of
the healing process.
Because patients are usually unaware of their refractive error, to help
determine if they are good candidates for refractive surgery it may be simplest
to ask if they can see the big E on the eye chart (even if it is a little
blurry). If they can, they are probably good can didates, although an eye
examination will be necessary to verify that they are. In general,
contraindications that apply to LASIK (ocular surface disease, keratoconus,
herpes simplex virus keratitis, rheumatoid arthritis, etc.) are relative
contraindications and also apply to Intacs. The best outcomes are with patients
whose keratometry readings are between 40 and 46 D.
The procedure does not treat astigmatism, although studies show that in
some patients, it may decrease astigmatism by about 0.50 D. Candidates should
have less than 1 D of astigmatism.
![[bar]](osnart/gradient.gif)
Advantages, disadvantages
 ---Correcting low
myopia: Intacs are implanted within the corneal stroma near the
periphery of the cornea. ---Correcting low
myopia: Intacs are implanted within the corneal stroma near the
periphery of the cornea.
The fact that the procedure does not correct astigmatism or high levels of
myopia is a disadvantage, although more than 50% of all myopes fit in the range
for the KeraVision procedure. Furthermore, it is relatively new technology with
only about 2,000 procedures performed worldwide. As an advantage, the surgical
treatment is performed in the peripheral cornea, not the central visual axis.
In addition, as shown in the accompanying topography, it does not reverse the
natural positive corneal asphericity. Another advantage is that Intacs are
removable or exchangeable. The second topography shows the cornea after
removal. Refractive error returns to the original, as does the corneal
topography.
With respect to time, this procedure will be as rapid to perform as current
laser procedures. Patients experience rapid visual recovery and, therefore,
little disturbance of their normal daily routines. Postoperative examinations
will be required, similar to the schedule of appointments required after LASIK.
With respect to cost, the Intacs are a completely different product. While the
cost is likely to be in line with PRK and LASIK, you can expect to see more
creativity in pricing and packaging.
For Your Information:
- Michael Twa, OD, director of patient care and clinical research for
keratorefractive surgery at the University of California – San Diego
Shiley Eye Center, can be contacted 9415 Campus Point Dr., La Jolla, CA
92093-0946; (619) 822-1805; fax: (619) 822-1514; e-mail:
mtwa@ucsd.edu. Dr. Twa has no direct
financial interest in the products mentioned in this article, nor is he a paid
consultant for any companies mentioned.
- For more information from KeraVision about Intacs, contact Mick Taylor at
(510) 353-3000.
|

