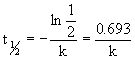Chapter 14: Chemical Kinetics
- chemical kinetics
area of chemistry dealing with speeds/rates of reactions
- rates of reactions affected by four factors
- 1) concentrations of reactants
- 2) temperature at which reaction occurs
- 3) presence of a catalyst
- 4) surface area of solid or liquid reactants and/or catalysts
14.1 Reaction Rates
- reaction rate speed of a chemical reaction
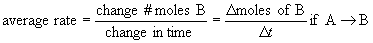
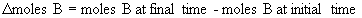

14.1.1 Rates in Terms of Concentrations
- rate calculated in units of M/s
- brackets around a substance indicate the concentration
- instantaneous rate
rate at a particular time
- instantaneous rate obtained from the straight line tangent that touches the curve at a specific point
- slopes give instantaneous rates
- instantaneous rate also referred to as the rate
14.1.2 Reaction Rates and Stoichiometry
- for the reaction
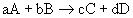
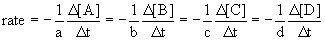
14.2 The Dependence of Rate on Concentration
- equation used only if C and D only substances formed
- Rate = k[A][B]
- Rate law
expression that shows that rate depends on concentrations of reactants
- K = rate constant
14.2.1 Reaction Order
- Rate = k[reactant 1]m[reactant 2]n
- m, n are called reaction orders
- m+n, overall reaction order
- reaction orders do not have to correspond with coefficients in balanced equation
- values of reaction order determined experimentally
- reaction order can be fractional or negative
14.2.2 Units of Rates Constants
- units of rate constant depend on overall reaction order of rate law
- for reaction of second order overall
- units of rate = (units of rate constant)(units of concentration)2
- units of rate constant = M-1s1
14.2.3 Using Initial Rates to Determine Rate Laws
- zero order no change in rate when concentration changed
- first order
proportional changes in rate
- second order
increase rate by 22 or 33, etc
- rate constant does not depend on concentration
14.3 The Changes of Concentration with Time
- rate laws can be converted into equations that give concentrations of reactants or products
14.3.1 First-Order Reactions
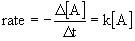
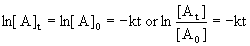
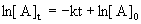
- corresponds to y = mx + b
- equations used to determine:
- 1) concentration of reactant remaining at any time
- 2) time required for given fraction of sample to react
- 3) time required for reactant concentration to reach a certain level
14.3.2 Half-Life
- half-life of first order reaction
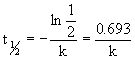
- half-life
time required for concentration of reactant to drop by one half initial value
- t1/2 of first order independent of initial concentrations
- half-life same at any given time of reaction
- in first order reaction concentrations of reactant decreases by ½ in each series of regularly spaced time intervals
14.3.3 Second-Order Reactions
- rate depends on reactant concentration raised to second power or concentrations of two different reactant each raised to first power
- Rate = k[A]2
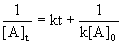
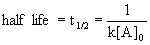
- half life dependent on initial concentration of reactant
14.4 Temperature and Rate
- chemiluminescent reaction reaction that produces light
- rate constant must increase with increasing temperature
14.4.1 The Collision Model
- collision model molecules must collide to react
- greater number of collisions the greater the reaction rate
- for most reactions only small amount of collisions lead to a reaction
14.4.2 Activation Energy
- Svante Arrhenius
- Molecules must have a minimum amount of energy to react
- Energy comes from kinetic energy of collisions
- Kinetic energy used to break bonds
- Activation energy, Ea minimum energy required to initiate a chemical reaction
- Activated complex or transition state atoms at the top of the energy barrier
- Rate depends on Ea
- Lower Ea means faster reaction
- Reactions occur with collisions and orientation of molecules
14.4.3 The Arrhenius Equation
- reaction rate data:
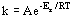 (Arrhenius Equation)
(Arrhenius Equation)- k = rate constant, Ea = activation energy, R = gas constant (8.314 J/mol K), T = absolute temperature, A = frequency factor
- A relates to frequency of collisions, favorable orientations
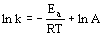
- ln k vs 1/t graph has slope Ea/R and y-intercept ln A
- for two temperatures:
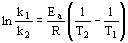
- used to calculate rate constant, k1 and T1
14.5 Reaction Mechanisms
- reaction mechanism process by which a reaction occurs
14.5.1 Elementary Steps
- elementary steps each step in a reaction
- molecularity
if only one molecule involved in step
- unimolecular
if only one molecule involved in step
- bimolecular
elementary step involving collision of two reactant molecules
- termolecular
elementary step involving simultaneous collision of three molecules
- elementary steps in multistep mechanism must always add to give chemical equation of overall process
- intermediate
product formed in one step and consumed in a later step
14.5.2 Rate Laws of Elementary Steps
- if reaction is known to be an elementary step then the rate law is known
- rate of unimolecular step is first order (Rate = k[A])
- rate of bimolecular steps is second order (Rate = k[A][B]
- first order in [A] and [B]
- if double [A] than number of collisions of A and B will double
14.5.3 Rate laws of multistep mechanisms
- rate-determining step slowest elementary step
- determines rate law of overall reaction
14.5.4 Mechanisms with an Initial First Step
- intermediates usually unstable, low and unknown concentrations
- whenever a fast step precedes a slow one, solve for concentration of intermediate by assuming that equilibrium is established in fast step
14.6 Catalysis
- catalyst substance that changes speed of chemical reaction without undergoing a permanent chemical change
14.6.1 Homogeneous Catalysis
- homogeneous catalyst catalyst that is present in same phase as reacting molecule
- catalysts alter Ea or A
- generally catalysts lowers overall Ea for chemical reaction
- catalysts provides a different mechanism for reaction
14.6.2 Heterogeneous Catalysis
- exists in different phase from reactants
- initial step in heterogeneous catalyst is adsorption
- adsorption
binding of molecules to surface
- adsorption occurs because ions/atoms at surface of solid extremely reactive
14.6.3 Enzymes
- biological catalysts
- large protein molecules with molecular weights 10,000 1 million amu
- catalase
enzyme in blood and liver that decomposes hydrogen peroxide into water and oxygen
- substrates
substances that undergo reaction at the active site
- lock-and-key model
substrate molecules bind specifically to the active site
- enzyme-substrate complex
combination of enzyme and substrate
- binding between enzyme and substrate involves intermolecular forces (dipole-dipole, hydrogen bonding, and London dispersion forces)
- product from reaction leaves enzyme allowing for another substrate to enter enzyme
- enzyme inhibitors
molecules that bind strongly to enzymes
- turnover number
number of catalyzed reactions occurring at a particular active site
- large turnover numbers = low activation energies