Chapter 15: Chemical Equilibrium
- chemical equilibrium – condition where the concentration of products and reactants do not change with time
15.1 The concept of Equilibrium
- at equilibrium kf[A] = kr[B]
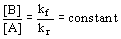
15.2 The Equilibrium Constant
- equilibrium condition can be reached from either forward or reverse direction
- Cato Maximillian Galdberg (1836-1902), and Peter Wauge (1833-1900)
- Law of mass action – relationship between concentrations of reactions and products at equilibrium
- If
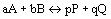 :
:
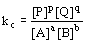 (equilibrium expression)
(equilibrium expression)- equilibrium expression depends only on stoichiometry of reaction and not mechanisms
- equilibrium constant:
- does not depend on initial concentrations
- does not matter if other substances present as long as they do not react with reactants or products
- varies with temperatures
- no units
15.2.1 Expressing Equilibrium Constants in Terms of Pressure, kp
15.2.2 The Magnitude of Equilibrium Constants
- k>>1; equilibrium lies to the right; products favored
- k<<1; equilibrium lies to the left; reactants favored
15.2.3 The Direction of the Chemical Equation and K
- equilibrium expression written in one direction is the reciprocal of the one in the other direction
15.3 Heterogeneous Equilibria
- homogeneous equilibria – substances in the same phase
- heterogeneous equilibria – substances in different phases
- concentration of pure liquid or solid
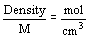
- density of pure liquid or solid is constant at any temperature
- if pure solid or liquid is involved in a reaction, its concentration is excluded from equilibrium expression
- pure solids must be present for equilibrium to be reached even through they are excluded from equilibrium expression
15.4 Calculating Equilibrium Constants
- determining unknown equilibrium concentrations
- 1) tabulate known initial and equilibrium concentrations
- 2) calculate change in concentration that occurs as system reaches equilibrium
- 3) use stoichiometry to determine change in concentration of unknown species
- 4) from initial concentrations and changes in concentrations, calculate equilibrium concentrations
15.4.1 Relating kc and kp
- PV = nRT; P = (n/V)RT = MRT
- PA = [A](RT)
- Kp=kc(RT)D
n
- D
n = change in moles from reactants to products
15.5 Applications of Equilibrium Constants
- equilibrium constant:
- 1) product direction reaction mixture will proceed
- 2) calculate concentrations of reactants and products once equilibrium is reached
15.5.1 Predicting the Direction of Reaction
- reaction quotient
- at equilibrium Q=k
- Q>k; reaction moves right to left
- Q<k; reaction moves left to right
15.5.2 Calculating of Equilibrium Concentrations
15.6 Le Châtelier’s Principle
- if system at equilibrium is disturbed by change in temperature, pressure or concentration then system will shift equilibrium position
15.6.1 Change in Reactant or Product Concentration
- addition of substance will result in consummation of part of added substance
- if substance removed, reaction will move to produce more of the substance
15.6.2 Effects of Volume and Pressure Changes
- reducing volume, reaction shifts to reduce number of gas molecules
- increase volume, reaction shifts to produce more gas molecules
- increase pressure, decrease volume reduces total number of moles
- pressure volume changes do not affect k as long as temperature is constant
- changes concentrations of gaseous substances
15.6.3 Effect on Temperature Change
- endothermic: reactants + heat «
products
- exothermic: reactants «
products + heat
- increase temperature, equilibrium shifts in direction that absorbs heat
- endothermic: increase T, increase k
- exothermic: increase T, decrease k
- cooling shifts equilibrium to produce heat
15.6.4 The Effect of Catalysts
- catalysts increase rate at which equilibrium is obtained
- does not change composition of equilibrium mixture