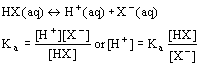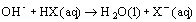Chapter 17: Additional Aspects of Aqueous Equilibria
17.1 The Common-Ion Effect
common-ion effect dissociation of a weak electrolyte decreases by adding a strong electrolyte to the solution that has a common ion with the weak electrolyte
17.2 Buffered Solutions
- buffers solutions that resist a change in pH
17.2.1 Composition and Action of Buffered Solutions
- buffers have both acidic and basic species to neutralize H+ and OH- ions
- acid dissociation equilibrium in buffered solution
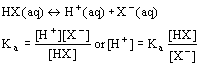
- pH determined by: 1) value of Ka 2) ration of [HX]/[X-]
- if OH- added:
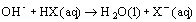
- [HX] decreases, [X-] increases
- if amounts of HX and X- are larger than amount of OH-
- ratio [HX]/[X-] doesnt change much, and pH is small
- when [HX] and [X-] are about the same, buffers are most effective
- [H+] equal to Ka
17.2.2 Buffer Capacity and pH
- buffer capacity amount of acid or base buffer can neutralize before the pH changes considerably
- capacity depends on amount of acid or base in buffer
- pH depends on Ka for acid and relative concentrations of the acid and base
- Henderson-Hasselbalch equation:
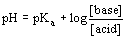
- [base] and [acid] = concentrations of conjugate acid-base pair
- when [base]=[acid], pH=Ka
- can use initial concentrations of acid and base components of buffer directly into equation
17.2.3 Addition of Strong Acids or Bases to Buffers
- reactions between strong acids and bases go to completion
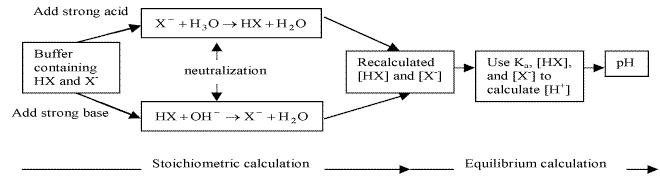
- strong base or acid assumed to be completely consumed by reaction with buffer if buffering capacity is not exceeded
17.3 Acid-Base Titrations
- solution containing a known [base] added to an acid or acid solution added to base
- acid-base indicators used to signal equivalence point
- titration curve pH vs Volume
17.3.1 Strong Acid Strong base Titrations
- pH starts out low ends high
- pH before equivalence point is pH of acid not neutralized by base
- pH at equivalence point is pH of solution
- pH equals 7.00
- for strong base titrations, the pH starts high ends low
17.3.2 The Addition of a Strong Base to a Weak Acid
- reaction between weak acid and strong base goes to completion
- calculating pH before equivalence point
- 1) stoichiometric calculations: allow strong base to react to completion producing a solution containing a weak acid and its conjugate base
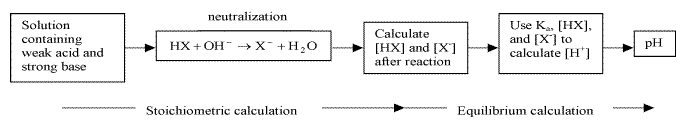
- 2) equilibrium calculation: use Ka and equilibrium expression to find equilibrium concentrations of the weak acid and its conjugate base, and H+
17.3.3 Titration Curves for Weak Acids or Weak Bases
- differences between strong acid-strong base titrations:
- 1) solution of weak acid as higher initial pH than solution of a strong acid with same concentration
- 2) solution of weak acid rises more rapidly in early part of titration and more slowly as it reached the equivalence point
- 3) pH is not 7.00 at equivalence point
- before equivalence point solution has mixture of weak acid and its salt
- also called the buffer region of curve
- at equivalence point solution contains only salt
- weakly basic due to hydrolysis of anion
- after equivalence point solution has mixture of salt and excess strong base
- pH determined by [base]
17.3.4 Titrations of Polyprotic Acids
- reaction occurs in series of steps
- titration curve shows multiple equivalence points
17.4 Solubility Equilibria
17.4.1 The Solubility-Product Constant, Ksp
- saturated solution dissolved and undissolved solute are at equilibrium
- expressed by g/L
- molar solubility moles of solute dissolved to form a liter of saturated solution (mol/L)
- Ksp equilibrium constant for the equilibrium between an ionic solid and its saturated solution
- Solubility of compound (g/L) à molar solubility of compound (mol/L) à [molar] of ions à Ksp of ions
17.5 Factors That Affect Solubility
- solubility affected by temperature and presence of other solutes
- solubility of ionic compound affected by:
- 1) the presence of common ions
- 2) pH of solution
- 3) presence of complexing agent
17.5.1 Common-Ion Effect
- solubility of slightly soluble salt decreases when a second solute has a common ion
17.5.2 Solubility and pH
- solubility of any ionic compound affected if solution is acidic or basic
- change only noticeable if both ions are moderately acidic or basic
- solubility of slightly soluble salts containing basic anions increase as [H+] increases (as pH is lowered)
- the more basic an anion is, the greater the solubility will be affected by pH
17.5.3 Formation of Complex Ions
- metal ions act as Lewis acids in water
- complex ion metal ion and Lewis base bonded together
- Kf formation constant, equilibrium expression for formation of a complex ion
- Solubility of metal salts increases in acceptable Lewis bases if metal forms a complex base
- Lewis bases NH3, CN-, OH-
17.5.4 Amphoterism
- amphoteric substances hydroxides, oxides of Al3+, Cr2+, Zn2+, and Sn2+
- dissolve in strongly basic solutions
- formation of complex anions containing, typically four, hydroxides bound to metal ion
- amphoterism also associated with behavior of water molecules that surround and bond to metal ions by Lewis acid-base interactions
17.6 Precipitation and Separation of Ions
- Q = ion product
- If Q > Ksp, precipitation occurs until Q = Ksp
- If Q = Ksp, equilibrium exists, have a saturated solution
- If Q < Ksp, solid dissolves until Q = Ksp
17.6.1 Selective Precipitation of Ions
- separation of ions in aqueous solution using a reagent that precipitates only with selected ions
17.7 Qualitative Analysis for Metallic Elements
- qualitative analysis determines presence or absence of a particular metal ion
- 1) ions separated into broad groups on basis of solubility
- 2) ions separated by dissolving selected members in group
- 3) ions identified by specific tests