- made of only hydrogen and carbon
- 4 types: alkanes, alkenes, alkynes, and aromatic hydrocarbons
- alkanes only single bonds
- also called saturated hydrocarbons
 have the largest amount of hydrogen atoms bonded to a single carbon atom
have the largest amount of hydrogen atoms bonded to a single carbon atom
Chapter 25: The Chistry of Life: Organic and Biological Chemistry
 have the largest amount of hydrogen atoms bonded to a single carbon atom
have the largest amount of hydrogen atoms bonded to a single carbon atom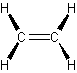 alkenes (olefins) have double carbon bonds
alkenes (olefins) have double carbon bonds![]()
|
Molecular Formula |
Condensed Structural Formula |
Name |
Boiling point (°C) |
|
CH4 |
CH4 |
Methane |
-161 |
|
C2H6 |
CH3 CH3 |
Ethane |
-89 |
|
C3H8 |
CH3 CH2 CH3 |
Propane |
-44 |
|
C4H10 |
CH3 CH2 CH2 CH3 |
Butane |
-0.5 |
|
C5H12 |
CH3 CH2 CH2 CH2 CH3 |
Pentane |
36 |
|
C6H14 |
CH3 CH2 CH2 CH2 CH2 CH3 |
Hexane |
68 |
|
C7H16 |
CH3 CH2 CH2 CH2 CH2 CH2 CH3 |
Heptane |
98 |
|
C8H18 |
CH3 CH2 CH2 CH2 CH2 CH2 CH2 CH3 |
Octane |
125 |
|
C9H20 |
CH3 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH3 |
Nonane |
151 |
|
C10H22 |
CH3 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH3 |
Decane |
174 |

Lewis Structure
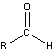 Aldehydes and Ketones
Aldehydes and Ketones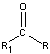
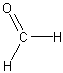
*formaldehyde*
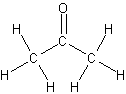
*Acetone*
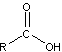
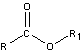
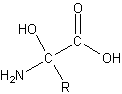
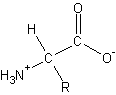
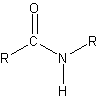 peptide bond
peptide bond 3) A nitrogen-containing organic base
3) A nitrogen-containing organic base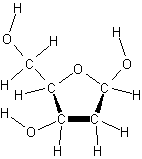
*ribose* *deoxyribose*
*molecular structures created from ChemSketch program*
*www.acdlabs.com*