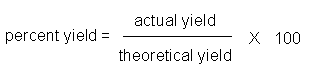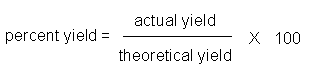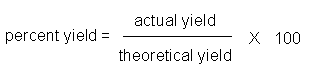Chapter 3: Stoichiometry: Calculations With Chemical Formulas and Equations
- atoms are neither created nor destroyed during any chemical reaction
- stoichiometry
– quantitative nature of chemical formulas and chemical reactions
3.1 Chemical Equations
- chemical equations – the way chemical reactions are represented
- reactants
– starting substances
- products
– substances produced from a reaction
- balanced equation
– equation with equal atoms on both sides of the equation
- subscripts should never be changed in balancing an equation
- coefficients changes only the amount and not identity of the substance
3.2 Patterns of Chemical Reactivity
3.2.1 Using the Periodic Table
- periodic table can be used to determine reactivity of substances
- all alkali metals react with water to form their hydroxide compounds and hydrogen
3.2.2 Combustion in Air
- rapid reaction that produces a flame
- most combustion reactions in air involve oxygen
- hydrocarbons and related compounds produce CO2 and H2O during combustion
3.2.3 Combination and Decomposition Reactions
- combination reactions two or more substances react to form one product
- decomposition reaction one substance produces two or more substances
3.3 Atomic and Molecular Weights
3.3.1 The Atomic Mass Scale
- atomic mass unit (amu)
– unit in measuring mass of atoms
- 1 amu = 1.66054*10-24g and 1 amu = 6.02214*1024amu
3.3.2 Average Atomic Masses
- atomic weight –
average atomic mass
3.3.3 Formula and Molecular Weights
- formula weight
– sum of the atomic weights of each atom in its chemical formula
- molecular weight
– same as formula weight
3.3.4 Percentage composition from Formulas
- ((atoms of element)(AW)/(FW of compound) * 100
3.3.5 The Mole
- avogadro’s number
– 6.02*1023 atoms
- molar mass
– numerically equal to its formula weight
- grams <use molar mass> moles <use avogadro’s number> molecules
3.5 Empirical Formulas from Analyses
- empirical formula gives relative number of atoms in each element
- mass % elements
>>> assume 100g sample >>> grams of each element >>> use atomic weights >>> moles of each element >>> calculate mole ratio >>> empirical formula
- "percent to mass, mass to mol, divide by small, multiply ‘til whole/"
3.5.1 Molecular Formula from Empirical Formula
- the subscripts in the molecular formula of a substance are always a whole-number multiple of the corresponding subscripts in its empirical formula
3.5.2 Combustion Analysis
3.6 Quantitative Information from Balanced Equations
- the coefficients in a balanced chemical equation can be interpreted both as the relative numbers of molecules involved in the reaction and as the relative numbers of moles
- stoichiometrically equivalent quantities
- grams reactant >> moles reactant >> moles product >> grams product
- grams of substance A
>> use molar mass of A >> moles of substance A >> use coefficients of A and B from balanced equation >> moles of substance B >> use molar mass of B >> grams of substance B
3.7 Limiting Reactants
- limiting reactant
– limits the amount of product formed
3.7.1 Theoretical Yields
- theoretical yield – the amount of product that is calculated to form
- actual yield – the amount of product actually formed