Chapter 4: Aqueous Reactions and Solution Stoichiometry
- aqueous solutions
– solutions in which water is the dissolving medium
4.1 Solution Composition
- solution
– homogeneous mixture of two or more substances
- solvent
– component that is present in greatest quantity
- solutes
– substances dissolved in the solvent
4.1.1 Molarity
- concentration
– the amount of solute dissolved in a given quantity of solvent or solution
- molarity
– number of moles of solute in a liter of solution
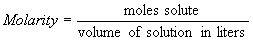
4.1.2 Dilution
- dilution
- obtaining a lower concentration of a solution by adding water
- moles solute before dilution = moles solute after dilution
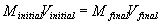
4.2 Properties of Solutes in Aqueous Solutions
- electrolyte
– substance whose aqueous solution contains ions
- nonelectrolyte – substance that does not form ions in solution
4.2.1 Ionic Compounds in Water
- dissociate
– when ions separate from a solid being dissolved
4.2.2 Molecular Comounds in Water
- the molecular structure is maintained
4.2.3 Strong and Weak Electrolytes
- strong electrolytes – ionic compounds that exists entirely of ions in solution
- weak electryolytes – molecular compounds that produce a small amound of ions
- chemical equilibrium – equilibrium of forming ions and recrystalizing ions
4.3 Acids, Bases, and Salts
4.3.1 Acids
- substances that ionize to form hydrogen ions
- proton donors
4.3.2 Bases
- substances that ionize to form hydroxide ions
4.3.3 Strong and Weak Acids and Bases
- strong acid, strong base
– strong electrolyte
- weak acid, weak base
– weak electrolyte
4.3.4 Neutralization Reactions and Salts
- neutralization reaction
– when an acid and base are mixed
- produces water and a salt
4.4 Ionic Equations
- molecular formula
– and equation written to show the complete chemical formulas of reactants and products
- spectator ions
– ions that do not play a role in a reaction
- net ionic equation
– equation where the spectator ions are removed
- only soluble strong electrolytes are written in ionic form
4.5 Metathesis Reactions
- 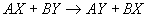
- for methathesis to occur:
- 1) the formation of an insoluble product
- 2) the formation of either a weak electrolyte or a nonelectrolyte
- 3) the formation of a gas that escapes from solution
4.5.1 Precipitation Reactions
- precipitate
– insoluble solid formed by a reaction in solution
- solubility
– amount of substance that can be dissolved in a given quantity
4.5.2 Solubility Guidelines for Ionic Compounds
- all common ionic compounds of the alkali metal ions and of the ammonium ion are soluble in water
4.5.3 Reactions in Which a Weak Electrolyte or Nonelectrolyte Forms
- hydrogen and hydroxide react to form water
- insoluble metal oxides react with acids
4.6 Introduction to Oxidation-Reduction Reactions
4.6.1 Reactions in Which a Gas Forms
- carbonates and bicarbonates
4.6.2 Oxidation and Reduction
- oxidation
– loss of electrons
- reduction
– gain of electrons
4.6.3 Oxidation of Metals by Acids and Salts
- whenever one substance is oxidized, some other substance must be reduced
- metals react with acids to form salts and hydrogen gas
4.6.4 The Activity Series
- activity series
– list of metals arranged in order of decreasing ease of oxidation
- active metals
– alkali metals and alkaline earth metals
- any metla on the list can be oxidized by ions of elements below it
4.7 Solution Stoichiometry and Chemical Analysis
4.7.1 Titrations
- statndard solution
– solution of known concentration
- titration
– a known solution that undergoes a specific chemical reaction of known stoichiometry with the solution of unknown concentration
- equivalence point
– stoichiometrically equivalent quantities of reactants are brought together
- indicator
– used to show the endpoint of the titration
![]()
![]()
![]()
![]()